4. DIAGNOSTIC EVALUATION
Tests are useful for diagnosis, monitoring, assessing the risk of disease progression, treatment planning, and predicting treatment outcomes. The clinical assessment of patients with LUTS has two main objectives:
- to identify the differential diagnoses, since the origin of male LUTS is multifactorial, the relevant EAU Guidelines on the management of applicable conditions should be followed;
- to define the clinical profile (including the risk of disease progression) of men with LUTS in order to provide appropriate care.
4.1. Medical history
The importance of assessing the patient’s history is well recognised [20-22]. A medical history aims to identify the potential causes and relevant co-morbidities, including medical and neurological diseases. In addition, current medication, lifestyle habits, emotional and psychological factors must be reviewed. The Panel recognises the need to discuss LUTS and the therapeutic pathway from the patient’s perspective. This includes reassuring the patient that there is no definite link between LUTS and prostate cancer (PCa) [23,24].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (see section 4.2) should be obtained to objectify and quantify LUTS [20,22]. Bladder diaries or frequency volume charts (FVC) are particularly beneficial (see section 4.3) [25]. Sexual function should also be assessed, preferably with validated symptom questionnaires such as the International Index of Erectile Function (IIEF) [26].
Summary of evidence | LE |
A medical history is an integral part of a patient’s medical evaluation. | 4 |
A medical history aims to identify the potential causes of LUTS as well as any relevant co-morbidities and to review the patient’s current medication and lifestyle habits. | 4 |
Recommendation | Strength rating |
Take a complete medical history from men with LUTS. | Strong |
4.2. Symptom score questionnaires
All published guidelines for male LUTS recommend using validated symptom score questionnaires [20,22]. Several questionnaires have been developed which are sensitive to symptom changes and can be used to monitor treatment [27-33]. Symptom scores are helpful in quantifying LUTS and in identifying which type of symptoms are predominant; however, they are not disease-, gender-, or age-specific. A systematic review (SR) evaluating the diagnostic accuracy of individual symptoms and questionnaires, compared with urodynamic studies (the reference standard), for the diagnosis of BOO in males with LUTS found that individual symptoms and questionnaires for diagnosing BOO were not significantly associated with one another [34].
4.2.1. The International Prostate Symptom Score (IPSS)
The IPSS is an eight-item questionnaire, consisting of seven symptom questions and one QoL question [28]. The IPSS score is categorised as ‘asymptomatic’ (0 points), ‘mildly symptomatic’ (1-7 points), ‘moderately symptomatic’ (8-19 points), and ‘severely symptomatic’ (20-35 points). Limitations include lack of assessment of incontinence, post-micturition symptoms, and bother caused by each separate symptom.
4.2.2. The International Consultation on Incontinence Questionnaire for Male LUTS (ICIQ-MLUTS)
The ICIQ-MLUTS was created from the International Continence Society (ICS) Male questionnaire. It is a widely used and validated patient completed questionnaire including incontinence questions and bother for each symptom [29]. It contains thirteen items, with subscales for nocturia and OAB, and is available in 24 languages. [35].
4.2.3. Danish Prostate Symptom Score (DAN-PSS)
The DAN-PSS [32] is a symptom score used mainly in Denmark and Finland. The DAN-PSS has twelve questions divided into parts A and B with questions on incontinence and measures the bother of each individual LUTS.
Summary of evidence | LE |
Symptom questionnaires are sensitive to symptom changes. | 3 |
Symptom scores can quantify LUTS and identify which types of symptoms are predominant; however, they are not disease-, gender-, or age-specific. | 3 |
Recommendation | Strength rating |
Use a validated symptom score questionnaire including bother and quality of life assessment during the initial assessment of male LUTS and for re-evaluation during and/or after treatment. | Strong |
4.3. Frequency volume charts and/or bladder diaries
The recording of the volume and time of each void by the patient is referred to as an frequency volume chart (FVC). Inclusion of additional information such as fluid intake, use of pads, activities during recording, or which grades symptom severity and bladder sensation is termed a bladder diary [5]. Parameters that can be derived from the FVC and bladder diary include day-time and night-time voiding frequency, total voided volume, the fraction of urine production during the night (NP index), and volume of individual voids.
The mean 24-hour urine production is subject to considerable variation. Likewise, circumstantial influence and intra-individual variation cause FVC parameters to fluctuate, though there is comparatively little data [36,37]. The FVC/bladder diary is particularly relevant in nocturia, where it underpins the categorisation of underlying mechanism(s) [38-40]. The use of FVCs may cause a ‘bladder training (BT) effect’ and influence the frequency of nocturnal voids [41].
The duration of the FVC/bladder diary needs to be long enough to avoid sampling errors, but short enough to avoid non-compliance [25]. A SR of the available literature recommended FVCs should continue for at least three days [42]. There is no data as to whether the three days should be consecutive or scattered or whether it has to be on weekdays or weekends, as long as it is representative. The ICIQ-Bladder diary (ICIQ-BD) is the only diary that has undergone full validation [43].
Summary of evidence | LE |
Frequency volume charts (FVC) and/or bladder diaries provide real-time documentation of urinary function and reduce recall bias. | 3 |
Three day FVCs provide reliable measurement of urinary symptoms in patients with LUTS similar to seven days and without losing the diagnostic accuracy. | 2b |
Recommendations | Strength rating |
Use a bladder diary to assess male LUTS with a storage component, especially nocturia. | Strong |
Tell the patient to complete a bladder diary for at least three days. | Strong |
4.4. Physical examination and digital-rectal examination
Physical examination particularly focusing on the suprapubic area, the external genitalia, the perineum, and lower limbs should be performed. Urethral discharge, meatal stenosis, phimosis, and penile cancer must be excluded.
4.4.1. Digital-rectal examination and prostate size evaluation
Digital-rectal examination (DRE) is the simplest way to assess prostate volume, but the correlation to prostate volume is poor. Quality-control procedures for DRE have been described [44]. Transrectal ultrasound (TRUS) is more accurate in determining prostate volume than DRE. There is an underestimation of prostate volume by DRE. The underestimation increases with increasing TRUS volume, particularly where the volume is > 30 mL [45]. A model of visual aids has been developed to help urologists estimate prostate volume more accurately [46]. One study concluded that DRE was sufficient to discriminate between prostate volumes > or < 50 mL [47].
Summary of evidence | LE |
Physical examination is an integral part of a patient’s medical evaluation. | 4 |
Digital-rectal examination can be used to assess prostate volume and texture; however, the correlation to actual prostate volume is poor. | 3 |
Recommendation | Strength rating |
Perform a physical examination including digital rectal examination in the assessment of male LUTS. | Strong |
4.5. Urinalysis
Urinalysis (dipstick or microscopy) must be included in the primary evaluation of any patient presenting with LUTS to identify conditions, such as urinary tract infections (UTI), microhaematuria and diabetes mellitus. If abnormal findings are detected further tests are recommended according to other EAU Guidelines, e.g., Guidelines on urinary tract cancers and urological infections [48-51].
Urinalysis is recommended in most Guidelines in the primary management of patients with
LUTS [52,53]. There is limited evidence, but general expert consensus suggests that the benefits outweigh the costs [54]. The value of urinary dipstick/microscopy for diagnosing UTI in men with LUTS without acute frequency and dysuria has been questioned [55].
Summary of evidence | LE |
Urinalysis (dipstick or microscopy) may indicate a UTI, proteinuria, haematuria, or glycosuria requiring further assessment. | 3 |
The benefits of urinalysis outweigh the costs. | 4 |
Recommendation | Strength rating |
Use urinalysis (by dipstick or microscopy) in the assessment of male LUTS. | Strong |
4.6. Prostate-specific antigen
4.6.1. Prostate-specific antigen and the prediction of prostatic volume
Pooled analysis of randomised controll trials (RCTs), of men with LUTS and presumed BPO, showed that prostate-specific antigen (PSA) has a good predictive value for assessing prostate volume, with areas under the curve (AUC) of 0.76-0.78 for various prostate volume thresholds (30 mL, 40 mL, and 50 mL). To achieve a specificity of 70%, whilst maintaining a sensitivity between 65-70%, approximate age-specific criteria for detecting men with prostate glands exceeding 40 mL are PSA > 1.6 ng/mL, > 2.0 ng/mL, and > 2.3 ng/mL, for men with BPH in their 50s, 60s, and 70s, respectively [56].
A strong association between PSA and prostate volume was found in a large community-based study in the Netherlands [57]. A PSA threshold value of 1.5 ng/mL could best predict a prostate volume of > 30 mL, with a positive predictive value (PPV) of 78%. The prediction of prostate volume can also be based on total and free PSA. Both PSA forms predict the TRUS prostate volume (± 20%) in > 90% of the cases [58,59].
4.6.2. Prostate-specific angtigen and the probability of PCa
The role of PSA in the diagnosis of PCa is presented by the EAU Guidelines on Prostate Cancer [60]. The potential benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient.
4.6.3. Prostate-specific antigen and the prediction of BPO-related outcomes
Serum PSA is a stronger predictor of prostate growth than prostate volume [61]. In addition, an RCT showed that PSA also predicted the changes in symptoms, QoL/bother, and maximum flowrate (Qmax) [62]. In a longitudinal study of men managed conservatively, PSA was a highly significant predictor of clinical progression to urinary retention [63,64]. In the placebo arms of large double-blind studies, baseline serum PSA predicted the risk of acute urinary retention (AUR) and BPO-related surgery [65,66]. An equivalent link was also confirmed by the Olmsted County Study. The risk for treatment was higher in men with a baseline PSA of > 1.4 ng/mL [67]. Patients with BPO seem to have a higher PSA level and larger prostate volumes. The PPV of PSA for the detection of BPO was recently shown to be 68% [68]. Furthermore, in an epidemiological study, elevated free PSA levels could predict clinical BPE, independent of total PSA levels [69].
Summary of evidence | LE |
Prostate-specific antigen has a good predictive value for assessing prostate volume and is a strong predictor of prostate growth. | 1b |
Baseline PSA can predict the risk of AUR and BPO related surgery. | 1b |
Recommendations | Strength rating |
Measure prostate-specific antigen (PSA) if a diagnosis of prostate cancer will change management. | Strong |
Measure PSA if it assists in the treatment and/or decision-making process. | Strong |
Counsel patients about PSA testing and the implications of a raised PSA test. | Strong |
4.7. Renal function measurement
Renal function may be assessed by serum creatinine or estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency or urinary retention are more prevalent in patients with signs or symptoms of BPO [70]. Even though BPO may be responsible for these complications, there is no conclusive evidence on the mechanism [71].
One study reported that 11% of men with LUTS had renal insufficiency [70]. Neither symptom score nor QoL was associated with the serum creatinine level. Diabetes mellitus or hypertension were the most likely causes of the elevated creatinine concentration. Comiter et al., [72] reported that non-neurogenic voiding dysfunction is not a risk factor for elevated creatinine levels. Koch et al., [73] concluded that only those with an elevated creatinine level or reduced eGFR require investigational ultrasound (US) of the kidney and bladder to assess post-void residual.
In the Olmsted County Study, there was a cross-sectional association between signs and symptoms of BPO (though not prostate volume) and chronic kidney disease (CKD) [74]. In 2,741 consecutive patients who presented with LUTS, decreased Qmax, a history of hypertension and/or diabetes were associated with CKD [75]. Another study demonstrated a correlation between Qmax and eGFR in middle-aged men with moderate-to-severe LUTS [76]. Patients with renal insufficiency are at an increased risk of developing post-operative complications [77].
Summary of evidence | LE |
Decreased Qmax and a history of hypertension and/or diabetes are associated with CKD in patients who present with LUTS. | 3 |
Patients with renal insufficiency are at an increased risk of developing post-operative complications. | 3 |
Recommendation | Strength rating |
Assess renal function if renal impairment is suspected based on history and clinical examination, or in the presence of hydronephrosis, or when considering surgical treatment for male LUTS. | Strong |
4.8. Post-void residual urine
Post-void residual (PVR) urine can be assessed by transabdominal ultrasound (US), bladder scan or catheterisation. Post-void residual is not necessarily associated with BOO, since high PVR volumes can be a consequence of obstruction and/or poor detrusor function/DU [78,79]. Using a PVR threshold of 50 mL, the diagnostic accuracy of PVR measurement has a PPV of 63% and a negative predictive value (NPV) of 52% for the prediction of BOO [80]. A large PVR is not a contraindication to watchful waiting (WW) or medical therapy, although it may indicate a poor response to treatment and especially to WW. In both the MTOPS and ALTESS studies, a high baseline PVR was associated with an increased risk of symptom progression [65,66].
Monitoring of changes in PVR over time may allow for identification of patients at risk of AUR [81]. This is of importance for the treatment of patients using antimuscarinic medication. In contrast, baseline PVR has little prognostic value for the risk of BPO-related invasive therapy in patients on α1-blockers or WW [82]. However, due to large test-retest variability and lack of outcome studies, no PVR threshold for treatment decision has yet been established; this is a research priority.
Summary of evidence | LE |
The diagnostic accuracy of PVR measurement, using a PVR threshold of 50 mL, has a PPV of 63% and a NPV of 52% for the prediction of BOO. | 3 |
Monitoring of changes in PVR over time may allow for identification of patients at risk of AUR. | 3 |
Recommendation | Strength rating |
Measure post-void residual in the assessment of male LUTS. | Weak |
4.9. Uroflowmetry
Urinary flow rate assessment is a widely used non-invasive urodynamic test. Key parameters are Qmax, voided volume, PVR, and flow pattern. Uroflowmetry parameters should preferably be evaluated with voided volume >150mL. As Qmax is prone to within-subject variation [83,84], it is useful to repeat uroflowmetry measurements, especially if the voided volume is < 150 mL, or Qmax or flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by threshold values. A threshold Qmax of 10 mL/s has a specificity of 70%, a PPV of 70% and a sensitivity of 47% for BOO. The specificity using a threshold Qmax of 15 mL/s was 38%, the PPV 67% and the sensitivity 82% [85]. If Qmax is > 15 mL/s, physiological compensatory processes mean that BOO cannot be excluded. Low Qmax can arise as a consequence of BOO [86], DU or an under-filled bladder [87]. Therefore, it is limited as a diagnostic test as it is unable to discriminate between the underlying mechanisms. Specificity can be improved by repeated flow rate testing.
Uroflowmetry can be used for monitoring treatment outcomes [88] and correlating symptoms with objective findings [85,89].
Summary of evidence | LE |
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by threshold values. Specificity can be improved by repeated flow rate testing. | 2b |
Recommendations | Strength rating |
Perform uroflowmetry in the initial assessment of male LUTS. | Weak |
Perform uroflowmetry prior to medical or invasive treatment. | Strong |
4.10. Imaging
4.10.1. Upper urinary tract
Men with LUTS are not at increased risk for upper tract malignancy or other abnormalities when compared to the overall population [73,90-92]. Several arguments support the use of renal US in preference to intravenous urography. Ultrasound allows for better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum, and simultaneous evaluation of the bladder, PVR and prostate, together with a lower cost, no radiation dose and less side effects [90]. Ultrasound can be used for the evaluation of men with large PVR, haematuria, or a history of urolithiasis.
Summary of evidence | LE |
Men with LUTS are not at increased risk for upper tract malignancy or other abnormalities when compared to the overall population. | 3 |
Ultrasound can be used for the evaluation of men with large PVR, haematuria, or a history of urolithiasis. | 4 |
Recommendation | Strength rating |
Perform ultrasound of the upper urinary tract in men with LUTS. | Weak |
4.10.2. Prostate
Imaging of the prostate can be performed by transabdominal US, TRUS, computed tomography (CT), and magnetic resonance imaging (MRI). However, in daily practice, prostate imaging is performed by transabdominal (suprapubic) US or TRUS [90].
4.10.2.1. Prostate size and shape
Assessment of prostate size is important for the selection of interventional treatment, i.e., open prostatectomy (OP), enucleation techniques, transurethral resection, transurethral incision of the prostate (TUIP), or minimally invasive therapies. It is also important prior to treatment with 5α-reductase inhibitors (5-ARIs). Prostate volume predicts symptom progression and the risk of complications [92].
Transrectal US is superior to transabdominal volume measurement [93,94]. The presence of a median lobe may guide treatment choice in patients scheduled for a minimally invasive approach since medial lobe presence can be a contraindication for some minimally invasive treatments (see section 5.3).
Summary of evidence | LE |
Assessment of prostate size by TRUS or transabdominal US is important for the selection of interventional treatment and prior to treatment with 5-ARIs. | 3 |
Recommendations | Strength rating |
Perform imaging of the prostate when considering medical treatment for male LUTS, if it assists in the choice of the appropriate drug. | Weak |
Perform imaging of the prostate when considering surgical treatment. | Strong |
4.10.3. Voiding cysto-urethrogram
Voiding cysto-urethrogram (VCUG), on its own, is not recommended in the routine diagnostic work-up of men with LUTS, but it may be useful for the detection of vesico-ureteral reflux, bladder diverticula, or urethral diseases and can be combined with urodynamics in the form of video-urodynamics. Retrograde urethrography may additionally be useful for the evaluation of suspected urethral strictures.
4.11. Urethrocystoscopy
Patients with a history of microscopic or gross haematuria, urethral stricture, or bladder cancer, who present with LUTS, should undergo urethrocystoscopy during diagnostic evaluation. The evaluation of a prostatic middle lobe with urethrocystoscopy should be performed when considering interventional treatments for which the presence of middle lobe may affect the treatment offered e.g., Urolift.
A prospective study evaluated 122 patients with LUTS using uroflowmetry and urethrocystoscopy [95]. The pre-operative Qmax was normal in 25% of 60 patients who had no bladder trabeculation, 21% of 73 patients with mild trabeculation and 12% of 40 patients with marked trabeculation on cystoscopy. All 21 patients who presented with diverticula had a reduced Qmax.
Another study showed that there was no significant correlation between the degree of bladder trabeculation (graded from I to IV), and the pre-operative Qmax value in 39 symptomatic men aged 53-83 years [96]. The largest study published on this issue examined the relation of urethroscopic findings to urodynamic studies in 492 elderly men with LUTS [97]. The authors noted a correlation between cystoscopic appearance (grade of bladder trabeculation and urethral occlusion) and urodynamic indices, DO and low compliance. It should be noted, however, that BOO was present in 15% of patients with normal cystoscopic findings, while 8% of patients had no obstruction, even in the presence of severe trabeculation [97].
Summary of evidence | LE |
Patients with a history of microscopic or gross haematuria, urethral stricture, or bladder cancer, who present with LUTS, should undergo urethrocystoscopy during diagnostic evaluation. | 3 |
No study clearly identified a strong association between the urethrocystoscopic and urodynamic findings. | 3 |
Recommendation | Strength rating |
Perform urethrocystoscopy in men with LUTS prior to minimally invasive/surgical therapies if the findings may change treatment. | Weak |
4.12. Urodynamics
In male LUTS, the most widespread invasive urodynamic techniques employed are filling cystometry and pressure flow studies (PFS). The major goal of urodynamics (UDS) is to explore the functional mechanisms of LUTS, to identify risk factors for adverse outcomes and to provide information for shared decision-making. Most terms and conditions (e.g., DO, low compliance, BOO/BPO, DU) are defined by urodynamic investigation.
4.12.1. Diagnosing bladder outlet obstruction
Pressure flow studies are used to diagnose and define the severity of BOO, which is characterised by increased detrusor pressure and decreased urinary flow rate during voiding. Bladder outlet obstruction/BPO has to be differentiated from DU, which exhibits decreased detrusor pressure during voiding in combination with decreased urinary flow rate [5].
Urodynamic testing may also identify DO. Studies have described an association between BOO and DO [98,99]. In men with LUTS attributed to BPO, DO was present in 61% and independently associated with BOO grade and ageing [98].
The prevalence of DU in men with LUTS is 11-40% [100,101]. Detrusor contractility does not appear to decline in long-term BOO and surgical relief of BOO does not improve contractility [102,103]. The UPSTREAM trial investigated whether urodynamics would reduce surgery without increasing urinary symptoms. UPSTREAM was a non-inferiority, RCT in men with bothersome LUTS, in whom surgery was an option, in 26 hospitals in England. In the UDS arm, 153/408 patients (38%) received surgery compared with 138/384 (36%) in the routine care (RC) arm. A total of 428 adverse events were recorded, with related events similar in both arms and eleven unrelated deaths. The UDS group was non-inferior to the RC group for IPSS, and UDS did not significantly reduce surgical rates. The authors concluded that routine use of UDS in the evaluation of uncomplicated LUTS has a limited role and should be used selectively [104]. If urodynamic investigation is performed, a rigorous quality control is mandatory [105,106].
Due to the invasive nature of the test, a urodynamic investigation is generally only offered if conservative and medical treatment have failed. The Guidelines Panel attempted to identify specific indications for UDS based on age, findings from other diagnostic tests and previous treatments. The Panel allocated a different degree of obligation for UDS in men > 80 years and men < 50 years, which reflects the lack of evidence. In addition, there was no consensus whether UDS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS and Qmax > 10 mL/s, although the Panel recognised that with a Qmax < 10 mL/s, BOO is likely and UDS is not necessarily needed.
Patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU Guidelines on Neuro-Urology [107].
4.12.2. Videourodynamics
Videourodynamics provides additional anatomical and functional information and may be recommended if the clinician considers this is needed to understand the pathophysiological mechanism of an individual patient’s LUTS.
Summary of evidence | LE |
Pressure-flow studies is not a test for routine use prior to prostate surgery for all patients | 3 |
Recommendations | Strength rating |
Perform pressure-flow studies (PFS) only in individual patients for specific indications prior to invasive treatment or when further evaluation of the underlying pathophysiology of LUTS is warranted. | Weak |
Perform PFS in men who have had previous unsuccessful (invasive) treatment for LUTS. | Weak |
Perform PFS in men considering invasive treatment who cannot void > 150 mL. | Weak |
Perform PFS when considering surgery in men with bothersome predominantly voiding LUTS and Qmax > 10 mL/s. | Weak |
Perform PFS when considering invasive therapy in men with bothersome, predominantly voiding LUTS with a post void residual > 300 mL. | Weak |
Perform PFS when considering invasive treatment in men with bothersome, predominantly voiding LUTS aged > 80 years. | Weak |
Perform PFS when considering invasive treatment in men with bothersome, predominantly voiding LUTS aged < 50 years. | Weak |
4.13. Non-invasive tests in diagnosing bladder outlet obstruction in men with LUTS
4.13.1. Prostatic configuration/intravesical prostatic protrusion
Prostatic configuration can be evaluated with TRUS, using the concept of the presumed circle area ratio (PCAR) [108]. The PCAR evaluates how closely the transverse US image of the prostate approaches a circular shape. The ratio tends toward one as the prostate becomes more circular. The sensitivity of PCAR was 77% for diagnosing BPO when PCAR was > 0.8, with 75% specificity [108].
Ultrasound measurement of intravesical prostatic protrusion (IPP) assesses the distance between the tip of the prostate median lobe and bladder neck in the midsagittal plane, using a suprapubically positioned US scanner, with a bladder volume of 150-250 mL; grade I protrusion is 0-4.9 mm, grade II is 5-10 mm and grade III is > 10 mm.
Intravesical prostatic protrusion correlates well with BPO (presence and severity) on urodynamic testing, with a PPV of 94% and a NPV of 79% [109]. Intravesical prostatic protrusion may also correlate with prostate volume, DO, bladder compliance, detrusor pressure at maximum urinary flow, BOO index and PVR, and negatively correlates with Qmax [110]. Furthermore, IPP also appears to successfully predict the outcome of a trial without catheter after AUR [111,112]. However, no information with regards to intra- or inter-observer variability and learning curve is yet available. Therefore, whilst IPP may be a feasible option to infer BPO in men with LUTS, the role of IPP as a non-invasive alternative to PFS in the assessment of male LUTS remains under evaluation.
4.13.2. Bladder/detrusor wall thickness and ultrasound-estimated bladder weight
For bladder wall thickness (BWT) assessment, the distance between the mucosa and the adventitia is measured. For detrusor wall thickness (DWT) assessment, the only measurement needed is the detrusor sandwiched between the mucosa and adventitia [113].
A correlation between BWT and UDS parameters has been reported. A threshold value of 5 mm at the anterior bladder wall with a bladder filling of 150 mL was best at differentiating between patients with or without BOO [114]. Detrusor wall thickness at the anterior bladder wall with a bladder filling > 250 mL (threshold value for BOO > 2 mm) has a PPV of 94% and a specificity of 95%, achieving 89% agreement with PFS [73]. Threshold values of 2.0, 2.5, or 2.9 mm for DWT in patients with LUTS are able to identify 81%, 89%, and 100% of patients with BOO, respectively [115].
All studies found that BWT or DWT measurements have a higher diagnostic accuracy for detecting BOO than Qmax or Qave of free uroflowmetry, measurements of PVR, prostate volume, or symptom severity. One study could not demonstrate any difference in BWT between patients with normal UDS, BOO or DO. However, the study did not use a specific bladder filling volume for measuring BWT [116]. Disadvantages of the method include the lack of standardisation, and lack of evidence to indicate which measurement (BWT/DWT) is preferable [117]. Measurement of BWT/DWT is therefore not recommended for the diagnostic work-up of men with LUTS.
Ultrasound-estimated bladder weight (UEBW) may identify BOO with a diagnostic accuracy of 86% at a cut-off value of 35 g [118,119]. Severe LUTS and a high UEBW (> 35 g) are risk factors for prostate/BPO surgery in men on α-blockers [120].
4.13.3. Non-invasive pressure-flow testing
The penile cuff method, in which flow is interrupted to estimate isovolumetric bladder pressure, shows promising data, with good test repeatability [121] and interobserver agreement [122]. A nomogram has also been derived [123] whilst a method in which flow is not interrupted is also under investigation [124].
The data generated with the external condom method [125] correlates with invasive PFS in a high proportion of patients [126]. Resistive index [127] and prostatic urethral angle [128] have also been proposed, but are still experimental.
4.13.4. The diagnostic performance of non-invasive tests in diagnosing bladder outlet obstruction in men with LUTS compared with pressure-flow studies
A SR including 42 studies investigated the diagnostic performance of non-invasive tests in diagnosing BOO in men with LUTS compared with UDS/PFS [129]. The majority of the included studies were prospective cohorts, and the diagnostic accuracy of the following non-invasive tests were assessed: penile cuff test; uroflowmetry; DWT/BWT; bladder weight; external condom catheter method; IPP; Doppler US; prostate volume/height; and near-infrared spectroscopy. Overall, although the majority of studies have a low risk of bias, data regarding the diagnostic accuracy of these non-invasive tests is limited by the heterogeneity of the studies in terms of the threshold values used to define BOO, the different urodynamic definitions of BOO used across different studies and the small number of studies for each test. It was found that specificity, sensitivity, PPV and NPV of the non-invasive tests were highly variable. Therefore, even though several tests have shown promising results regarding non-invasive diagnosis of BOO, invasive urodynamics remains the modality of choice.
Summary of evidence | LE |
Data regarding the diagnostic accuracy of non-invasive tests is limited by the heterogeneity of the studies as well as the small number of studies for each test. | 1a |
Specificity, sensitivity, PPV and NPV of the non-invasive tests were highly variable. | 1a |
Recommendation | Strength rating |
Do not offer non-invasive tests as an alternative to urodynamics/pressure-flow studies for diagnosing bladder outflow obstruction in men. | Strong |
4.14. Novel assessment
4.14.1. Visual prostate symptom score
A novel visual prostate symptom score (VPSS) has been prospectively tested vs. the IPPS and correlated positively with the IPSS score [130,131]. This visual score can be used as an option in men with limited literacy.
4.14.2. Micro-RNA
The use of miR-221 has been shown to have the potential to be used as a biomarker and novel target in the early diagnosis and therapy of BPH [132].
Figure 2: Assessment algorithm of LUTS in men aged 40 years or older
Readers are strongly recommended to read the full text that highlights the current position of each test in detail.
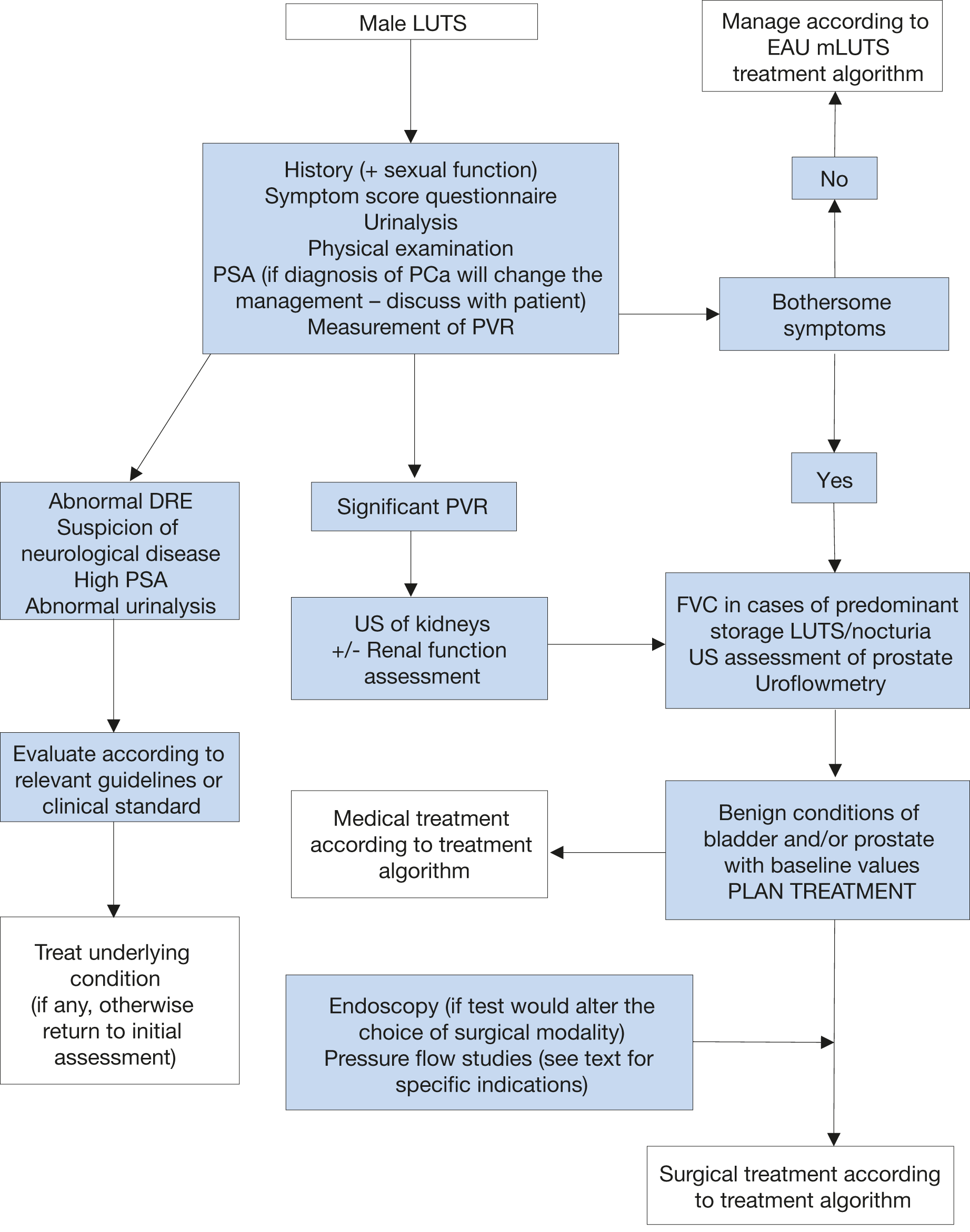 DRE = digital-rectal examination; FVC = frequency volume chart; LUTS = lower urinary tract symptoms; PCa = prostate cancer; PSA = prostate specific antigen; PVR = post-void residual; US = ultrasound.
DRE = digital-rectal examination; FVC = frequency volume chart; LUTS = lower urinary tract symptoms; PCa = prostate cancer; PSA = prostate specific antigen; PVR = post-void residual; US = ultrasound.