7. DISEASE MANAGEMENT
7.1. Stage I germ cell tumours
7.1.1. Germ cell neoplasia “in situ” (GCNIS)
If GCNIS is diagnosed and the contralateral testis is normal, options include orchidectomy or close observation, as the five-year risk of developing TC is 50% [101]. In a solitary testis, local radiotherapy (18-20 Gy in fractions of 2 Gy) should be considered [94,102-104]. Radiotherapy to a solitary testis will result in infertility and increased long-term risk of Leydig cell insufficiency [94]. Fertile patients who wish to father children may defer radiation therapy and be monitored with regular testicular US [64].
Chemotherapy is ineffective to reliably irradicate GCNIS [105,106].
7.1.2. Seminoma germ cell tumour clinical stage I
Up to 20% of seminoma germ cell tumour clinical stage I (SGCT CS I) patients have subclinical metastatic disease, usually in the retroperitoneum, and will relapse after orchidectomy alone [107,108]. Adjuvant treatment decisions should be based on thorough discussions with the patient, incorporating potential risks and benefits, as well as individual patient circumstances. Regardless of management, survival in stage I disease should be almost 100%.
7.1.2.1. Surveillance
This requires a strict protocol of repeated cross-sectional imaging, monitoring of serum tumour markers and clinical assessment for the early identification of patients experiencing relapse who must receive salvage treatment (See Table 11).
Several prospective, non-randomised surveillance studies have been conducted over the past decade. These have shown an overall risk of relapse in unselected CS I patients of 12-20% at five years, with 17% in the largest series of over 1,500 patients [109]. Most occur in the retroperitoneum during the first two years [110,111].
Risk of relapse is 12% with small size (tumours < 3 cm) as a single parameter [33,110]. With both, small tumour size (< 4 cm) and absence of stromal rete testis invasion, even lower recurrence rates of 6% are described.
The cancer-specific survival (CSS) rate on “active surveillance” for CS I seminoma is over 99% [109,111,112]. Whilst cost effective compared to other management strategies [113], surveillance can represent a burden to the patient due to the need for repeated imaging of the retroperitoneum and clinic visits. These may impact patient compliance which is crucial to an active surveillance strategy.
7.1.2.2. Adjuvant chemotherapy
A randomised controlled trial (RCT) comparing one cycle of carboplatin reaching area under curve of 7 mg/mL/min (AUC 7) with adjuvant RT, showed no difference in relapse-free rates (95% and 96%), time to recurrence and survival after a median follow-up of four years [114]. Adjuvant carboplatin (AUC 7) is therefore an alternative to RT or surveillance in CS I seminoma [114]. Time to relapse after Carboplatin may be longer than with active surveillance, as retrospective data reported a median time to relapse of nineteen months, with 15% of relapses occurring beyond three years. Most patients relapsing after adjuvant carboplatin can be successfully treated by standard, stage-adapted cisplatin-based chemotherapy [115].
7.1.2.3. Adjuvant radiotherapy
Radiotherapy should generally be reserved for a highly selective group of patients, who would be unsuitable for systemic chemotherapy in the event of relapse. This relates to the toxicity of RT, and specifically the long-term risk of non-germ cell malignancies in the radiation field [116-119].
7.1.2.4. Risk-adapted treatment
Tumour size > 4 cm and stromal rete testis invasion may stratify patients into low- and high-risk groups (please refer to chapter 4.3). Prospective trials based on these risk factors have demonstrated the feasibility of a risk-adapted approach [31-34,120].
A trial of 897 patients offered surveillance to patients with no, or one risk factor, whilst patients with both risk factors were offered one dose of carboplatin, AUC 7 [34]. At a median follow-up of 5.6 years, in the patients without risk factors, 4% of surveillance patients relapsed, compared to 2% receiving adjuvant carboplatin. With one or both risk factors, 15.5% of surveillance patients relapsed compared to 9% receiving adjuvant carboplatin. Thirty-three per cent of relapses after adjuvant carboplatin occurred more than three years after orchidectomy with 3% occurring after five years [34].
7.1.2.5. Guidelines for the treatment of clinical stage I seminoma testis tumours
Summary of evidence | LE |
Patients with stage I seminoma have, in general, a low risk of recurrence | 2a |
Primary testicular tumour size correlates with the risk of relapse. Stromal rete testis invasion and other factors such as vascular invasion or epididymal invasion show a less reliable correlation with the risk of relapse. | 2a |
The evidence to guide adjuvant treatment decisions upon risk factors is, however, still too limited to justify routine use in clinical practice. | 2a |
Active surveillance is a feasible approach with conditional relapse risk in unselected series of between 12-20%. | 2a |
In patients without risk factors, the five-year relapse rate under surveillance is 4-6%, whereas in the | 2b |
In non-randomised prospective series five-year relapse rates with adjuvant carboplatin are 2% in patients without risk factors and 9% in patients with one or both risk factors. | 2b |
Adjuvant chemotherapy with one course carboplatin AUC 7 is not inferior to adjuvant radiotherapy when pathological risk factors are considered. Relapse rates with both adjuvant treatments are around 5%. | 1b |
Adjuvant radiotherapy is associated with an increased risk of developing secondary non-germ cell malignancies. | 2b |
Recommendations | Strength rating |
Fully inform the patient about all available management options, including surveillance or adjuvant therapy after orchidectomy, as well as treatment-specific recurrence rates and acute and long-term side effects. | Strong |
Offer surveillance as the preferred management option if resources are available and the patient is compliant. | Strong |
Offer one dose of carboplatin at area under curve (AUC) 7 if adjuvant chemotherapy is considered. | Strong |
Do not perform adjuvant treatment in patients at very low-risk of recurrence (no risk factors). | Strong |
Do not routinely perform adjuvant radiotherapy. | Strong |
Adjuvant radiotherapy should be reserved only for highly selected patients not suitable for surveillance and with contraindication for chemotherapy. | Strong |
7.1.3. Non-seminomatous germ cell tumours clinical stage I
Management options for non-seminomatous germ cell tumours clinical stage I (CS I-NSGCTs) include surveillance and adjuvant chemotherapy. Retroperitoneal lymph node dissection (RPLND) has a limited role in selected cases. Overall, approximately 70% of CS I-NSGCTs are cured with orchidectomy alone. In those with the high-risk feature of LVI, historical figures reported relapse in 50% compared to 15% in those without LVI. A thorough discussion should be undertaken with the patient outlining the potential advantages and disadvantages of treatment options, as well as individual co-morbidities, disease features, risk factors, specific circumstances, and personal preferences, to guide their treatment decision.
7.1.3.1. Surveillance
Surveillance for CS I-NSGCT entails a strict protocol of repeated cross-sectional imaging, monitoring of serum tumour markers and clinical assessment for the early identification of patients experiencing relapse who must receive salvage treatment (See Table 12).
The largest reports of surveillance indicate a cumulative relapse risk in about 30% of CS I-NSGCT (five-year conditional risk of relapse 42%, and 17% for high- and low-risk CS I-NSGCT, respectively) [108,109]. Of these, 92% present within the first two years [108,109,121-123].
7.1.3.2. Retroperitoneal lymph node dissection
Since the introduction of cisplatin based chemotherapy the role of adjuvant RPLND in men with stage I GCT has decreased. According to data from high-volume and expert centres, primary RPLND is associated with a risk of relapse < 15% [124]. More recent data report a relapse rate of 10% in case of negative nodes (pathologic stage -PS – I) and < 30% in case of nodal metastases (PS II) [124-126], as a consequence possibly due to selection or stage migration. The few indications in stage I disease include men with teratoma with somatic malignant component, or patients who are not willing or suitable to undergo chemotherapy in case of recurrence, in particular in those when vascular invasion is present.
The presence of LVI, predominant embryonal carcinoma, primary pT stage and extranodal tumour extension histologically all appear associated with an increased risk of recurrence. The use of these further parameters has yet to be clearly defined in clinical practice [125-127]. With PS II, adjuvant chemotherapy comprising two cycles of (B)EP is the standard option. A recent publication supports the safety of surveillance alone, as about 80% are relapse free at two and five years and those with relapse can be rescued with standard chemotherapy [128,129].
Strategies to reduce the morbidity of primary RPLND include nerve-sparing and minimally invasive approaches. In a multicentre setting, higher rates of in-field recurrences and complications have been reported with nerve-sparing RPLND [130,131]. This suggests that primary RPLND, when indicated or chosen, should be performed by an experienced surgeon in a specialist centre. Minimally invasive (laparoscopic or robot-assisted) primary RPLND, appears feasible and safe (e.g., low-complication rate) in experienced hands. This must only be performed in high-volume RPLND centres with appropriate minimal-invasive surgery expertise [132-139].
Despite some advantages; including good efficacy; a less-demanding and costly follow-up due to the reduced need for cross-sectional imaging [140], RPLND for CS I-NSGCT has diminished its role in view of the high CSS rates of surveillance, the low relapse rates with adjuvant chemotherapy, and the lower reproducibility of primary RPLND on a large scale.
7.1.3.3. Adjuvant chemotherapy
Adjuvant chemotherapy has been evaluated with both one and two cycles of BEP in CS I-NSGCT. A prospective trial from 1996, as well as subsequent studies, used two cycles of BEP in high-risk patients (LVI present) [141-143]. In these series, including 200 patients, some with a median follow-up of nearly 7.9 years [141], a relapse rate of only 2.7% was reported, with minimal long-term toxicity. Two cycles of cisplatin-based adjuvant chemotherapy does not appear to adversely affect fertility or sexual activity [144].
Other studies have shown one cycle of adjuvant BEP results in similar very low recurrence rates (2-3%) [145,146]. Reduction from two to one cycle of BEP improves the risk-benefit ratio of adjuvant chemotherapy considerably. A randomised phase III trial has also compared two-year recurrence free survival with adjuvant BEP x 1 to RPLND. Results favoured chemotherapy with recurrence free survival of 99.5% vs. 91% [131]. No clinically relevant differences in quality of life (QoL) were detected [147].
A community based prospective study of 490 unselected patients with CS I-NSGCT that received adjuvant single cycle BEP had five-year relapse rates of 3% and 2% for LVI+ and LV- patients, respectively. After a median follow-up of eight years these rates were sustained, no relapses were observed beyond 3.3 years [145,146]. These numbers imply that > 90% of relapses are prevented by single cycle BEP which is now the recommended strategy if adjuvant chemotherapy is considered [145,146]. The very-long term (> 20 years) side effects of adjuvant chemotherapy, particularly cardiovascular, are yet to be fully defined and this should be considered during decision-making [148,149].
Limited data are available on outcomes with relapse after adjuvant BEP. A retrospective analysis indicated that about one third of these relapses were late and that the outcome may be slightly worse compared to those presenting with de novo metastatic disease [150].
7.1.3.4. Risk-adapted treatment
A risk-adapted strategy is an alternative to any single approach for patients with CS I-NSGCT. The advantages and disadvantages of treatment options must be discussed with patients in the context of their specific circumstances including disease risk factors, co-morbidities, and personal preference, as well as clinician recommendation in reaching a treatment decision. Lympho-vascular invasion is the strongest and most reproducible predictive factor for relapse and should be carefully outlined to the patient to assist in their decision-making.
Patients without LVI should be guided to consider surveillance, although some patients with significant co-morbidities or concerns regarding salvage chemotherapy with multicycle cisplatin-based chemotherapy may opt for adjuvant therapy. Those with LVI should have their high risk of relapse (up to 50%) highlighted and be guided to consider adjuvant management, and chemotherapy with BEP x 1 as the “preferred” option.
Some patients may wish to consider primary RPLND, although they need to be aware of the potential additional requirement of adjuvant chemotherapy if nodes contain active disease (pN1), as well as the 10% risk of systemic relapse, even if pN0, requiring subsequent chemotherapy treatment (BEP x 3).
7.1.3.5. Post-pubertal teratoma with somatic malignant component
A multi-institutional study analysing retrospective datasets of CS I patients with post-pubertal teratoma with somatic malignant component (TSMC) suggested these patients had inferior five-year OS of approximately 10% compared to other CS I-GCT patients. Furthermore, CS I TSMC cases undergoing primary RPPLND had a much higher proportion of nodal metastases (PS II) than expected (37.5%). Despite its limitations, this study provides the only evidence on this issue and supports primary RPLND in CS I-NSGCT with TSMC [151].
For patients presenting with CS I pure post-pubertal teratoma without a somatic malignant component, surveillance provides comparable survival outcomes to primary RPLND [152]. However, subtype discrepancies in primary diagnostics of post-pubertal teratoma are not infrequent and consist in addition of subtype and involve secondary somatic type of malignancy in 83% of cases. As such, central review by an expert genitourinary pathologist is recommended when teratoma is diagnosed in the orchidectomy specimen [153].
7.1.3.6. Guidelines for the treatment of clinical stage I non-seminoma testis
Summary of evidence | LE |
Lymphovascular invasion increases the risk of relapse. | 2a |
The relapse rate with active surveillance is up to 50%, depending on LVI status. | 2a |
The relapse rate in patients who receive adjuvant chemotherapy with BEP (x 1 cycle) is up to 3%. | 2a |
Adjuvant chemotherapy with BEP is superior to adjuvant RPLND in terms of the risk of relapse. | 1b |
A risk-adapted approach, based on lymphovascular invasion is feasible. | 2b |
The acute toxicity of one cycle adjuvant BEP is low. | 1b |
Recommendations | Strength rating |
Inform patients about all management options after orchidectomy: surveillance, adjuvant chemotherapy, and retroperitoneal lymph node dissection, including treatment-specific recurrence rates as well as acute and long-term side effects. | Strong |
Offer surveillance or risk-adapted treatment based on lymphovascular invasion (see below). | Strong |
Discuss one course of cisplatin, etoposide, bleomycin as an adjuvant treatment alternative in patients with stage I non-seminomatous germ cell tumour if patients are not willing to undergo or comply with surveillance. | Strong |
7.1.3.7. Risk-adapted treatment for clinical stage I non-seminomatous germ cell tumour based on vascular invasion
Recommendations | Strength rating |
Stage IA (pT1, no vascular invasion): low-risk | |
Offer surveillance if the patient is willing and able to comply. | Strong |
Offer adjuvant chemotherapy with one course of cisplatin, etoposide, bleomycin (BEP) in low-risk patients not willing (or unsuitable) to undergo surveillance. | Strong |
Stage IB (pT2-pT4): high-risk | |
Offer adjuvant chemotherapy with one course of BEP, or surveillance and discuss the advantages and disadvantages. | Strong |
Offer surveillance to patients not willing to undergo adjuvant chemotherapy. | Strong |
Offer nerve-sparing retroperitoneal lymph node dissection to highly selected patients only; those with contraindication to adjuvant chemotherapy and unwilling to accept surveillance. | Strong |
Primary retroperitoneal lymph node dissection should be advised in men with post-pubertal teratoma. | Weak |
Figure 1: Risk-adapted treatment in patients with clinical stage I non-seminoma NSGCT [42]*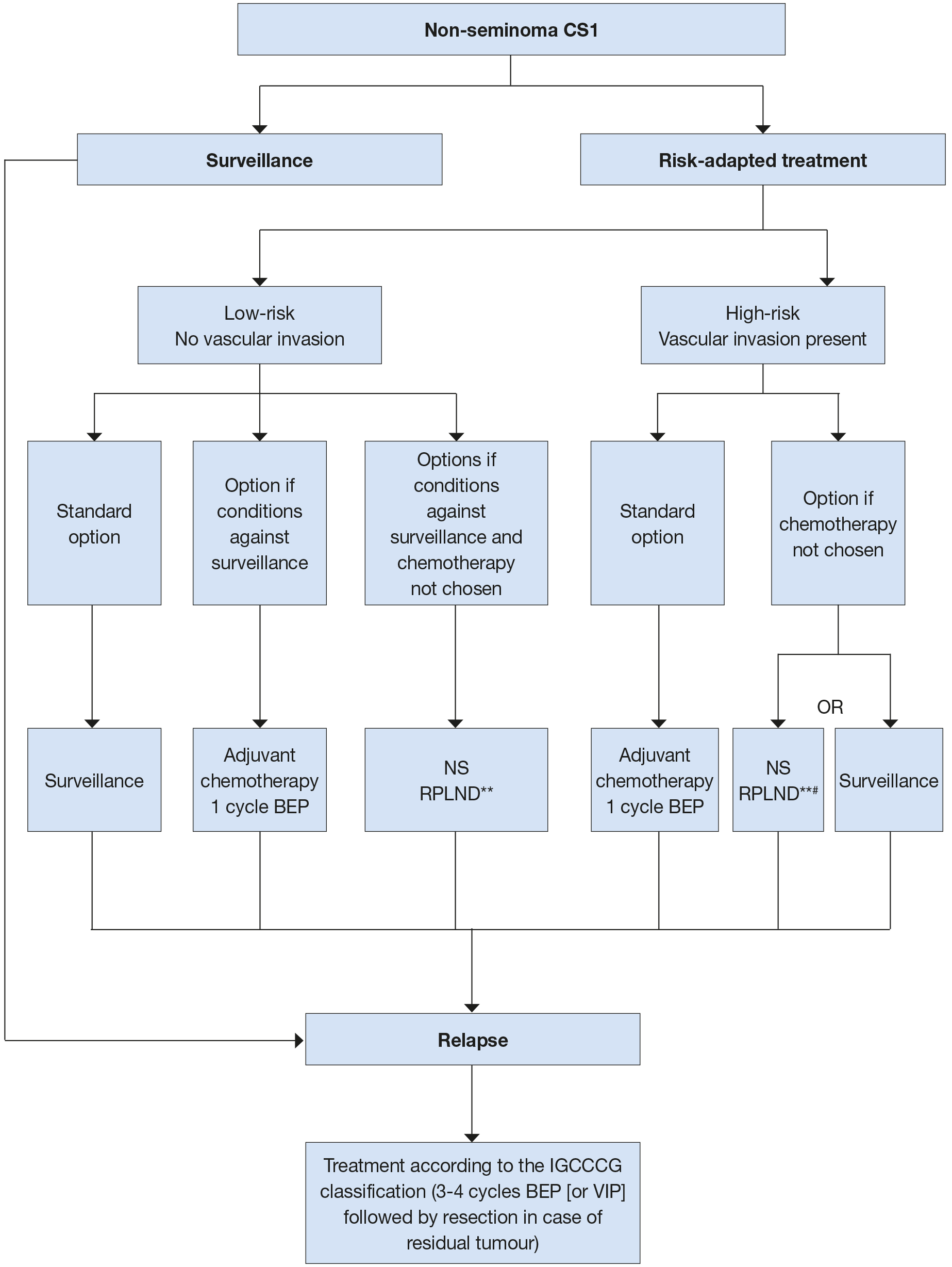 * Discuss all treatment options with individual patients, to allow them to make an informed decision as to their further care.
* Discuss all treatment options with individual patients, to allow them to make an informed decision as to their further care.
** In case of PS II, the rate of recurrence is higher and chemotherapy can be administered (max. 2 cycles).
# Primary retroperitoneal lymph node dissection should be advised in men with post-pubertal teratoma with somatic malignant component.
BEP = cisplatin, etoposide, bleomycin; CS = clinical stage; IGCCCG = International Germ Cell CancerCollaborative Group; NS = nerve-sparing; RLNPD = retroperitoneal lymph node dissection; VIP = etoposide,cisplatin, ifosfamide.
7.2. Metastatic germ cell tumours
The first-line treatment of metastatic GCTs depends on:
- the histology of the primary tumour;
- prognostic groups as defined by the IGCCCG (Table 3) [41];
- serum tumour marker decline at the end of the first cycle of chemotherapy in poor-prognosis patients.
In relapsed patients, a prognostic score has been developed including response to first-line therapy which can be used to estimate patient outcome following salvage chemotherapy [154].
7.2.1. Clinical stage I with (persistently) elevated serum tumour markers
If AFP or β-hCG increase or fail to normalise following orchidectomy, US examination of the contralateral testicle must be performed. If a contralateral tumour is excluded, repeat staging four weeks after orchidectomy is required [42].
Some patients may have stable but slightly elevated AFP or β-HCG and can be initially monitored. Treatment should be commenced if markers rise or when follow-up imaging demonstrates metastatic disease [42].
The treatment of true CS IS-NSGT should be the same as other metastatic non-seminoma. With this, five- and ten-year disease-free survival of 87% and 85%, respectively, have been reported [155].
7.2.2. Metastatic disease (stage IIA/B)
7.2.2.1. Stage IIA/B seminoma
Patients with enlarged retroperitoneal lymph nodes < 2 cm in greatest diameter and normal markers may be observed for six to eight weeks with repeat-staging imaging as these may be non-metastatic. Treatment should only be initiated if metastatic disease is unequivocal, based on biopsy, increasing nodal size/number, or subsequent marker rise [42,155]. A special case are those patients who can undergo primary RPLND within a trial or institutional study (see below for further details).
Standard historical treatment of stage II A/B seminoma has been radiotherapy, with reported relapse rates of 9-24% [156,157]. The radiation dose recommended in stage IIA and IIB is 30 Gy and 36 Gy, respectively, with the standard field encompassing the para-aortic (PA) and ipsilateral iliac nodes. With these, five-year relapse-free survival rates in stage IIA and IIB are 92% and 90%, respectively [156,157]. Reduced dose to 27 Gy in stage IIA is associated with higher relapse rate of 11% [111].
Chemotherapy is the alternative option for stage II seminoma. The standard regimen in good-risk seminoma is BEP x 3 (Table 6) or EP x 4 if bleomycin is contraindicated [158]. There are no randomised studies comparing radiotherapy and chemotherapy. A meta-analysis of thirteen high-quality studies, comparing efficacy and toxicity of radiotherapy and chemotherapy showed that these appeared similarly effective in both stage IIA/IIB patients, although with a non-significant trend towards greater efficacy for chemotherapy (HR: 2.17) in stage IIB seminoma [159]. Acute toxicity was almost exclusively reported following chemotherapy, while long-term toxicity was more frequent following radiotherapy, mainly comprising bowel toxicity and secondary cancers, generally in the irradiated field [159]. Several series have shown an increased risk of developing a second solid cancer of 1.8-2.0 with radiotherapy [160]. Long term toxicities of chemotherapy including secondary cancers are also a concern [160].
Currently, cisplatin is the preferred option given the concerns with long term effects of radiotherapy and second malignancy. Radiation therapy stands as an equal option therapy, although best suited for patients who are elderly, have contraindications or difficulties tolerating systemic chemotherapy.
Primary RPLND has also been reported for CS II seminoma [161,162]. Data from the National Cancer Data Base identified 155 men who underwent primary RPLND for CS II A/B reporting five-year OS of 92%. Specific trials are addressing the role of primary RPLND compared to standard options. These remain immature and insufficient to recommend primary RPLND in stage II seminoma.
Figure 2: Treatment options in patients with seminoma clinical stage IIA and B*
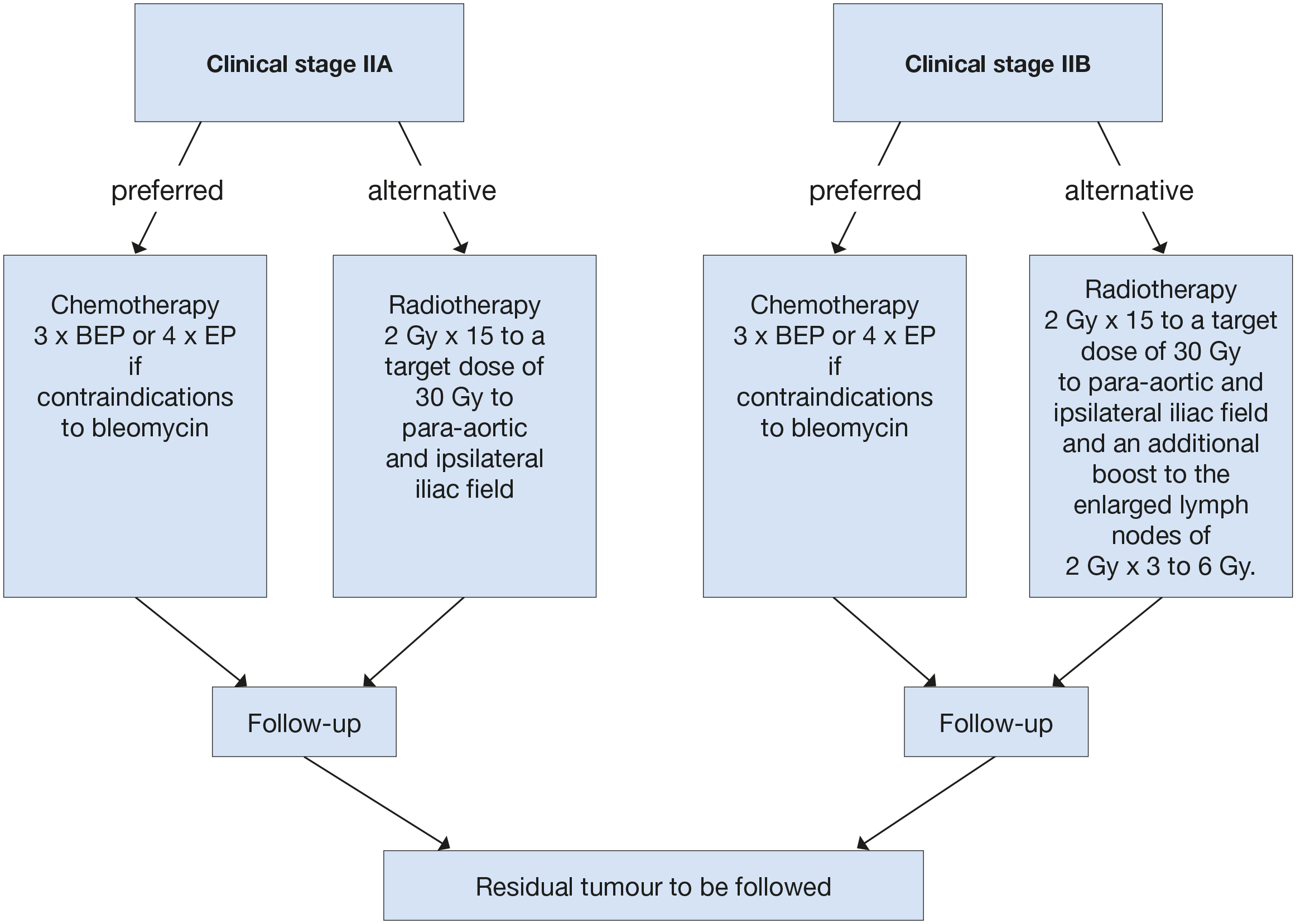
*When enlarged retroperitoneal lymph nodes are < 2 cm and with normal markers, treatment should not be initiated unless metastatic disease is unequivocal based on biopsy, increasing nodal size/number, or subsequent marker rise.
BEP = cisplatin, etoposide, bleomycin; EP = etoposide, cisplatin.
7.2.2.2. Stage II A/B non-seminoma (NSGCT)
Treatment of stage II A/B non-seminoma (NSGCT) should be treated as outlined in figure 3.
Initial surveillance may be considered in patients with normal markers and equivocal lymph nodes (< 2 cm) and requires early re-evaluation at six weeks. A shrinking mass may be observed further. If the lesion progresses or fails to resolve it should be regarded and treated as CS II.
7.2.2.2.1. Marker Positive
Patients with elevated tumour markers and radiological stage IIA/B at diagnosis or relapse should be treated with chemotherapy as outlined in tables 7.1 and 7.2. and section 7.2.3.1 based on IGCCCG risk group. Most patients will be good prognosis for whom BEP x 3 is most appropriate or EP x 4 if there are concerns with the use of bleomycin.
Primary RPLND for CS IIA/B disease with elevated markers is not recommended outside a specific study in a referral centre [163,164].
7.2.2.2.2. Marker Negative
With CS IIA NSGCT disease and normal or normalized tumour markers, nerve-sparing RPLND performed by an experienced surgeon in a specialized center is the recommended initial treatment. Patients may be downstaged to PSI in up to 20% of cases and require no further treatment. Patients with post-pubertal teratoma alone will avoid unnecessary chemotherapy as surgery alone is curative.
The oncological outcomes after RPLND in CSII NSGCT have been evaluated in a recent systematic review [165]. Of the included studies the majority were retrospective and, in these studies, the included patients differed substantially in histopathology of the testicular primary, size and number of retroperitoneal lymph nodes resected, surgical templates, and the use of adjuvant chemotherapy. Among men with marker negative CSII NSGCT, PS2 2 is confirmed in 80%. Without adjuvant chemotherapy 12–40% recurred compared to 0–4% in those who received adjuvant chemotherapy.
These findings align with large single centre reports of outcomes following RPLND alone for PSII NSGCT with active disease [121,128,129,166]. These studies reported five year relapse of less than 30%, with the majority occurring outside the retroperitoneum requiring systemic chemotherapy according to risk group.
Adjuvant chemotherapy may be discussed with the patient to reduce the risk of relapse in this setting. Key issues include risk factors for relapse (as positive lymph node-ratio), the risk of overtreatment in up to 70% of cases and the need of rigorous follow-up. When adjuvant chemotherapy is chosen, standard treatment is BEP for a maximum of two cycles, although a recent single-centre study of 150 patients undergoing two cycles EP following RPLND and PS II disease reported a ten-year relapse-free survival of 98% [167].
When a possible marker negative stage II A/B relapse is diagnosed more than two years after initial diagnosis, CT- or US-guided biopsy is advised to confirm the diagnosis of GCT relapse before initiating treatment. An RPLND is the alternative option and should be performed if biopsy is not feasible or does not provide confirmation of active disease. There is insufficient published data on FDG-PET scans in this situation to provide any recommendation.
Figure 3: Treatment options in patients with non-seminoma clinical stage IIA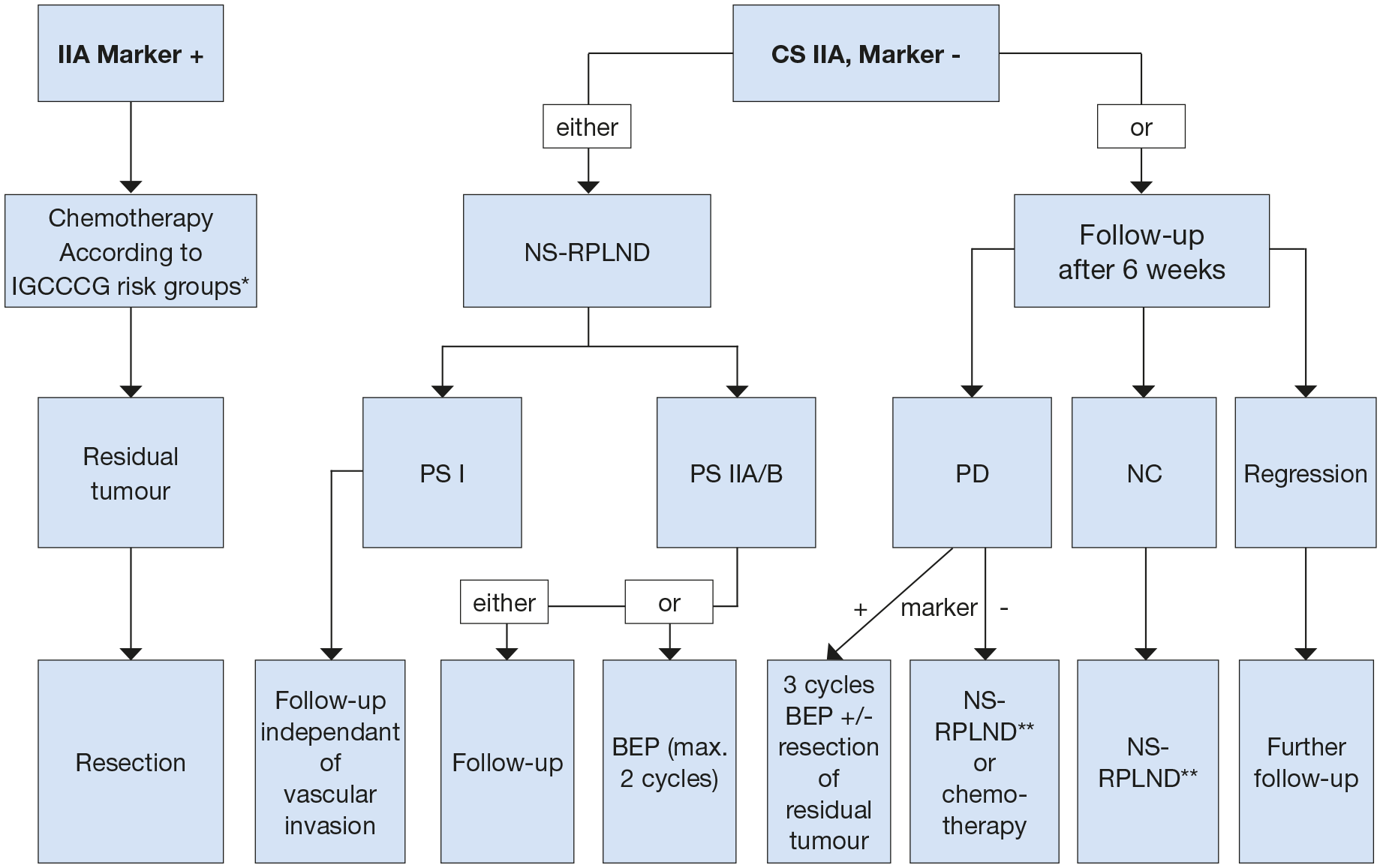 * Most of the patients will be good prognostic group (BEP x3 or PE x4).
* Most of the patients will be good prognostic group (BEP x3 or PE x4).
** In case of PS II A/B patient can be followed-up or receive adjuvant chemotherapy (maximum of 2 cycles).
BEP = cisplatin, etoposide, bleomycin; NS = nerve-sparing; RPLND = retroperitoneal lymph node dissection; PS = pathological stage; PD = progressive disease; NC = no change.
7.2.3. Metastatic disease (stage II C and III)
7.2.3.1. Primary chemotherapy
7.2.3.1.1. Good-prognosis risk group - seminomatous germ cell tumour
For metastatic seminoma, a cisplatin-based regimen should be used [168]. Cisplatin-based combination chemotherapy has shown superior efficacy over carboplatin-based regimens [168]. The standard regimen in good-risk seminoma is three, twenty-one day cycles of BEP (Table 6). Alternatively, etoposide, cisplatin (EP) x 4 may be considered especially when bleomycin is contraindicated [169]. This achieves similar response rates but may have a slightly higher risk of relapse [170].
Post-chemotherapy masses should be managed as described in Section 7.5.2.
7.2.3.1.2. Intermediate-prognosis risk group - seminomatous germ cell tumour
For patients with intermediate-risk seminoma, BEP x 4 is the standard regimen. If bleomycin is contraindicated bleomycin, etoposide, cisplatin, ifosfamide (VIP) should be given. No RCT has focused specifically on this rare group of patients (see Table 3).
7.2.3.1.3. Good-prognosis risk group - non-seminomatous germ cell tumour
The standard regimen in good-risk non-seminoma is BEP x 3 (Table 6) [41,169].
An RCT supports the equivalence of three- or five-day regimes with three or four cycles of BEP for projected two-year PFS. Three-day regimes are associated with increased toxicity [171,172]. Based on these data, the BEP x 3 as a five-day regimen is strongly recommended in the good-prognosis risk group.
Table 6: Cisplatin, etoposide, bleomycin (BEP) regimen (interval 21 days)
Drug | Dosage | Duration of cycles |
Cisplatin | 20 mg/m2 | Days 1-5* |
Etoposide | 100 mg/m2 | Days 1-5 |
Bleomycin | 30 mg | Days 1, 8, 15 |
*Plus, hydration.
Two RCTs support the superiority of BEP X 3 over other regimes or schedule intensities [158,173]. A further RCT has suggested that when EP is used, the mortality rate is twice that with BEP, although the difference did not reach statistical significance [158].
Patients with a clear contraindication to bleomycin may receive EP x 4 [171]. In all other cases omission of bleomycin is not recommended.
Chemotherapy should be given without reduction of the doses at 21-day intervals. Cytopenias on day fifteen of BEP are common although bleomycin should still be given on day fifteen irrespective of neutropenia or thrombocytopenia. Delaying a chemotherapy cycle is justified only in the presence of severe granulocytopenia < 500/mm3 or thrombocytopenia < 50,000/IU. Mild neutropenia without signs of infection alone is not a reason to delay the next cycle. As Granulocyte colony-stimulating factor (G-CSF) lowers the risk of neutropenic sepsis, its up-front use may be considered in selected patients at risk, even though it is not a standard prophylaxis with BEP. Granulocyte colony-stimulating factor, must be given if neutropenic infections occur during or after chemotherapy, or when treatment is delayed due to myelotoxicity [174].
7.2.3.1.4. Intermediate-prognosis risk group - non-seminomatous germ cell tumour
The standard regimen is BEP x 4 [175]. Four cycles of VIP has similar efficacy, but is more myelotoxic [176]. Four cycles of VIP including primary G-CSF prophylaxis should be applied in patients with contraindications to bleomycin.
7.2.3.1.5. Poor-prognosis risk group - non-seminomatous germ cell tumour
The standard regimen is four cycles of BEP. Four cycles of VIP have similar efficacy, but is more myelotoxic [176]. Several RCTs have shown no advantage in OS for upfront high-dose chemotherapy (HDCT) in the overall poor-prognosis patient group [177,178].
Serum tumour marker decline is the only prospectively confirmed predictor for response to cisplatin chemotherapy in metastatic germ cell tumour patients. Patients with inadequate tumour marker decline after the first or second cycle represent a prognostically inferior subgroup [178,179]. There are several ways to calculate tumour marker decline kinetics with an example available at: https://www.gustaveroussy.fr/calculation-tumor/NSGCT.html.
An RCT demonstrated improved PFS when intensifying treatment with dose-dense chemotherapy in patients with an early unfavourable tumour marker decline [180]. The trial was not powered to estimate OS differences. Based on the results from this trial, patients with an unfavourable tumour marker decline after BEP x 1 can be switched to a more intensive (dose-dense) chemotherapy regimen [180]. Additional patient groups with an unfavourable prognosis on standard treatment are those with primary mediastinal non-seminoma and patients with brain metastases at initial diagnosis [107,181]. These may also be candidates for upfront intensified treatment, preferably in a prospective study.
In RCTs, primary HDCT with subsequent autologous stem cell transplantation has not shown an OS benefit in the poor-prognosis patient population [177,178]. Selected patients, however, may derive a benefit from primary HDCT with three consecutive cycles of high-dose VIP. A specific group are those with primary mediastinal non-seminomas for whom prognosis with relapse is extremely poor [182]. Better outcomes are reported for patients with intermediate or poor prognosis when treated at high-volume centres, where optimal multidisciplinary treatment strategies can be defined. [183-185]. Due to unfavourable survival poor-prognosis patients should be treated in ongoing prospective trials or registries, whenever possible.
There are no general recommendations for treatment modifications for patients with poor performance status (Karnofsky < 50%) or extended liver infiltration (> 50%), although two small patient series indicate that an initial cycle of dose-reduced therapy may reduce acute mortality without compromising long-term outcomes. The number of subsequent cycles of full-dose therapy should, however, not be reduced after an initial low-dose induction cycle [184,186].
Patients with widespread pulmonary metastases are at risk for pulmonary haemorrhage and subsequent acute respiratory distress syndrome (ARDS) with induction chemotherapy. To reduce this risk, primary cytoreductive induction chemotherapy with EP over two to three days should be administered, followed by the first cycle of standard chemotherapy when the risk of ARDS has passed (typically after ten days) [184]. Bleomycin should be omitted in patients where subsequent lung surgery for residual mass resection is likely.
Table 7: Level of evidence for prognostic group and treatment
Prognostic group IGCCCG | Treatment | LE |
Good (SGCT and NSGCT) | BEP x 3 or EP x 4 | 1b |
Intermediate (SGCT and NSGCT) | BEP x 4 or VIP x 4 | 1b |
Poor (NSGCT) | BEP x 4 or VIP x 4 if favourable marker decline | 1b |
Dose escalation in selected cases with inadequate serum tumour marker decline | 1b |
7.2.3.1.6. Prevention of thromboembolism events during chemotherapy
Some RCTs have assessed the risks and benefits of thromboprophylaxis in ambulatory cancer patients receiving chemotherapy and report a relative risk reduction of 30-60% in venous thromboembolic events (VTE) but a doubling of bleeding risk [187-190]. Based on these results, the most recent ASCO Clinical Practice Guideline Update recommends thromboprophylaxis with apixaban, rivaroxaban, or low molecular weight heparin (LMWH) to cancer patients with a high risk of VTE and low risk of bleeding [191]. Metastatic germ cell tumour (mGCT) patients were under-represented in all trials; thus, it is not clear whether this recommendation applies to this group although retrospective data suggests a similar efficacy of VTE prophylaxis [192].
The EAU Guideline panel has discussed a recommendation regarding thromboprophylaxis. All members agreed that men with mGCTs undergoing chemotherapy are at high-risk for VTE, and with the exception of those with choriocarcinoma and high volume extra-peritoneal disease, are at low-risk of bleeding. Given the apparent high VTE incidence and only non-validated VTE risk factors, the panel preferences were divided between those panel members that favoured thromboprophylaxis in all men and those panel members that restricted thromboprophylaxis to men with certain risk factors. Additionally, the majority of the panel agreed that a central venous-access device should be avoided whenever possible as this represents the only modifiable risk factor, which remained significantly associated with VTE in a multivariable risk-prediction model [193,194].
*For more information regarding the prevention of thromboembolism events during chemotherapy, please see appendix 2, available online https://uroweb.org/guidelines/testicular-cancer/publications-appendices.
7.2.3.1.7. Guidelines for the Prevention of thromboembolism events during chemotherapy
Summary of evidence | LE |
Thromboembolic events occur more frequently in male patients with GCTs receiving chemotherapy than in young males under chemotherapy for other cancers. | 2b |
Retrospective studies have identified multiple risk factors for the development of thromboembolic events including increasing stage, size of retroperitoneal lymph nodes at different cut-offs, Khorana score > 3 and indwelling vascular access device (only modifiable risk factor). | 2b |
Recommendations | Strength rating |
Balance the individual patients’ potential benefits and risks of thromboprophylaxis during first-line chemotherapy in men with metastatic germ cell tumours. | Weak |
Avoid use of central venous-access devices during first-line chemotherapy whenever possible. | Weak |
7.3. Treatment evaluation and further treatment
7.3.1. Treatment evaluation
Response to treatment should be assessed after the initial induction cycle by repeat imagining and/or re-evaluation of tumour markers. With marker decline and/or radiologically regressing or stable tumour features, the planned chemotherapy should be completed [194,195]. If markers decline, but metastases progress on imaging, induction therapy must be completed [196]. If markers have normalised and masses with features of post-pubertal teratoma progress early surgical resection should be considered.
Slow marker-decline with the initial one to two cycles of chemotherapy warrants consideration for dose intensification (see section 7.2.3.1.5 re https://www.gustaveroussy.fr/calculation tumor/NSGCT.html).
Following completion of treatment, cases with a low-level β-hCG plateau should be observed to determine whether complete normalisation subsequently occurs. In patients with a low plateau serum AFP level after chemotherapy, removal of residual masses should be undertaken, with subsequent AFP monitoring. Salvage chemotherapy is only indicated for documented marker progression [196,197].
7.3.2. Residual tumour resection
7.3.2.1. Seminoma
A residual mass of seminoma should initially be monitored with imaging and tumour markers [198-200].
As FDG-PET has a high NPV, in patients with residual masses > 3 cm in largest diameter, this should be considered in order to provide more information on disease viability [201-203]. It should not be performed until at least two months after completion of chemotherapy, as inflammation and the desmoplastic reaction induced by chemotherapy may result in a false positive result [52]. The NPV for active disease is > 90% which can be reassuring [201,202]. In contrast PPV ranges from 23-69% and thus caution is advised on initiating active therapy driven only by positive findings on FDG-PET-CT [204].
When a post-chemotherapy mass remains positive at reclassification with FDG-PET with no volume increase, repeat FDG-PET should be performed six weeks later. A recent publication shows a low PPV for vital tumours in residual lesions (generally > 3 cm) after chemotherapy in metastatic seminoma (11-38% depending on sub-group). Therefore, caution is recommended with FDG-PET as a single parameter to drive clinical decisions in a persistent mass [204]. In patients with progressive disease on radiological criteria (i.e., a growing mass which enhances with CECT or is FDG-PET avid), salvage therapy is indicated [205-207].
Patients with persistently high and/or progressing β-hCG elevation after first-line chemotherapy should proceed to salvage chemotherapy. Progressing patients without β-hCG progression should undergo histological verification (e.g., by percutaneous or surgical biopsy) before salvage chemotherapy is given. When RPLND is indicated, this should be performed in referral centres, as residual seminoma masses may be extremely difficult to remove due to intense fibrosis [206].
7.3.2.2. Non-seminoma
Following first-line BEP only 6-10% of residual masses contain active cancer, 50% post-pubertal teratoma, and 40% necrotic-fibrotic tissue only [208]. Restaging patients following chemotherapy with FDG-PET is not indicated [50-52]. With complete radiological remission, RPLND is not indicated [209,210].
Usual timing for restaging is three to four weeks after the beginning of the last cycle. No diagnostic or risk calculator can accurately predict histology of the residual masses. Thus, resection is mandatory in all patients with a residual mass > 1 cm in greatest diameter at cross-sectional CECT imaging until novel predictive models are externally validated [211-214]. Surgery, when indicated, should be performed within six to eight weeks after the last chemotherapy cycle.
The role of surgery with residual retroperitoneal lesions < 1 cm is uncertain. It is difficult to distinguish between a true residual node below 10 mm and a complete remission, and many authors consider these situations as equivalent. Residuals containing cancer or teratoma are possible, but the vast majority of patients have fibro-necrotic tissue only [215]. Whilst post-chemotherapy RPLND with residuals < 10 mm or complete remission is an option [216], the alternative option is close surveillance with recurrence risk of 6-9% depending on the follow-up duration [209,210]. In the series with the longest follow-up of 15.5 years, twelve (9%) of 141 patients relapsed despite a complete response following primary treatment [210]. Eight of the twelve relapsing patients were cured with subsequent treatment. These cases should be discussed on individual basis considering the orientation and expectations of the patient.
Residual masses after salvage chemotherapy or HDCT in first or subsequent salvage situations have a greater risk of active disease [217]. Surgery is therefore indicated even with residual masses < 1 cm [209,210].
When resection is indicated, bilateral nerve sparing RPLND is the standard option. Ipsilateral template resection avoids contralateral nerve dissection and may be considered for residuals with a diameter < 5 cm [218], as well as unilateral lymph node metastases on pre- and post-chemotherapy CT scans, left-sided tumours only require para-aortic resection whereas right-side tumours need paracaval and inter-aortocaval resection down to the iliac arteries [219,220]. Mapping studies indicate a potential risk of contralateral disease with this approach [221]. The mere resection of the residual tumour (so called lumpectomy) should not be performed [210,214,215,217,218,220,222].
Laparoscopic or robotic RPLND may yield comparable outcomes to open procedures in selected cases with low-volume residual disease and when undertaken by very-experienced surgeons. This should only be considered in specialist TC centres with expertise in open RPLND and minimally invasive surgery to ensure appropriate case selection. In this setting, up to 30% of post-Chemotherapy RPLND have been reported via a laparoscopic approach [223-225]. Experience with robot-assisted laparoscopic RPLND, and specifically long term outcome remains limited [226]. Atypical recurrences have been reported, and occur more often with this approach [133].
7.3.3. Sequencing of surgery in the case of multiple sites
In general, surgery should commence at the site with the highest volume of residual disease. The histology of the mass diverges in different organ sites [211]. In cases of residual retroperitoneal and lung masses, the presence of fibro-necrotic tissue in the retroperitoneum is associated with a probability of as high as 90%, that lung masses contain the same histology [227]. When pathologic examination of the lesions from the initial side show complete necrosis observation may be considered when there are multiple contralateral tumours for whom resection may be challenging. Discordant histology between lung sites, however, may occur in up to 20% of cases and thus patients in this situation should be closely monitored with reconsideration of surgery or biopsy if radiological features change [228,229].
7.3.3.1. Quality and intensity of surgery
Resection of visceral structures and/or major vessels, requiring vascular reconstruction/replacement may be required to achieve radical resection and patients undergoing adjunctive complex surgery have a greater risk of complications [230,231]. In patients with intermediate- or poor-risk and residual disease > 5 cm, the probability of vascular procedures is as high as 20% [232]. These cases must therefore be referred to specialised centres capable of interdisciplinary surgery (hepatic resections, vessel replacement, spinal neurosurgery, thoracic surgery). Even with centralisation of treatment, the median number of RPLNDs performed per surgeon/year in the U.K. is six [233]. Nevertheless, patients treated within such centres benefit from a significant reduction in peri-operative mortality from 6-0.8% [234]. In addition, specialised urologic surgeons are capable of reducing the local recurrence rate from 16-3% with a higher rate of complete resections [235].
7.3.3.2. Salvage and desperation surgery
Surgery of resectable disease after salvage treatment remains a potentially curative option in patients with any residual mass following salvage chemotherapy. Survival after surgery and first salvage chemotherapy improved by 70% at ten years, following taxane-containing regimens [236]. Even with extensive salvage chemotherapy, surgery remains a fundamental tool to achieve durable complete remissions in up to 20% of patients [237,238].
Desperation surgery refers to resection of non-responsive or progressive (e.g., rising markers) disease following salvage chemotherapy. When the disease is resectable, a significant proportion of these patients can be rendered disease-free in the long term [239].
7.3.3.3. Consolidation chemotherapy after secondary surgery
After resection of necrosis or post-pubertal teratoma, no further treatment is required. With incomplete resection of viable cancer, two adjuvant cycles of conventionally dosed cisplatin-based chemotherapy may be given in certain subgroups (e.g., poor-prognosis patients) [222]. Caution is required with cumulative doses of bleomycin. With complete resection of active disease, comprising < 10% of the total volume of the mass, particularly in patients who initially had a good-prognosis based on IGCCCG criteria, the relapse rate is very low and adjuvant chemotherapy is not beneficial in preventing further relapse [240]. The prognosis is worse if malignant disease is present in masses resected after second- and third-line chemotherapy, although further chemotherapy is not indicated [241].
7.3.4. Systemic salvage treatment for relapse or refractory disease
Cisplatin combination salvage chemotherapy will result in long-term remissions in approximately 50% of patients who relapse after first-line chemotherapy. These results are highly dependent on several prognostic factors [242]. The regimens of choice are four cycles of a three-agent regimen including cisplatin and ifosfamide plus a third drug: VIP, paclitaxel (TIP), or potentially gemcitabine (GIP) (Table 8) [243,244]. No RCT has compared these regimens. Due to their potential risk of lethal haematological toxicity, these regimens should be used with G-CSF support and by well-trained oncologists.
Table 8: Standard VIP, TIP and GIP salvage chemotherapy (interval 21 days)
Regimen | Chemotherapy agents | Dosage | Duration of cycles |
VIP | Cisplatin Etoposide Ifosfamide | 20 mg/m2 75-100 mg/m2 1.2 g/m2 | Days 1-5 Days 1-5 Days 1-5 |
TIP | Paclitaxel Ifosfamide Cisplatin | 250 mg/m2 xx 1.5 g/ m2 25 mg/m2 | 24 hour continuous infusion day 1 Days 2-5 Days 2-5 |
Alternative schedule | |||
Paclitaxel Ifosfamide Cisplatin* | 175 mg/m2 1.2 g/m2 20 mg/m2 | Day 1, 3 hour infusion Days 1-5 Days 1-5 | |
GIP | Gemcitabine Ifosfamide Cisplatin | 1000 mg/m2 1200 mg/m2 20 mg/m2 | Day 1 + 5 Days 1-5 Days 1-5 |
xx An MRC schedule uses paclitaxel at 175 mg/m2 in a 3 hour infusion [244].
A retrospective analysis by the International Prognostic Factors Study Group (IPFSG) evaluated the risk of relapse in patients in whom this occurred after at least three cisplatin cycles and subsequent cisplatin conventional-dose or carboplatin-based high-dose salvage chemotherapy [154]. Seven variables: histology, primary tumour location, response, progression-free interval after first-line treatment and level of AFP, hCG and the presence of liver, bone or brain metastasis at salvage treatment, were identified as independent prognostic variables of relapse after initial cisplatin chemotherapy [154]. Using these factors, five risk-groups: very low-risk = -1 points; low-risk = 0 points; intermediate-risk = 1-2 points; high-risk = 3-4 points; and very high-risk > 5 points; were identified with significant differences in PFS and OS. Table 9 illustrates these five risk groups and the corresponding two-year PFS and three-year OS rates [154]. Several recent trials have validated this scoring system [245-248]. As in first-line therapy, the prognostic impact of tumour marker decline applies in the salvage setting [249]. While progression to induction chemotherapy was negative for OS, prior use of paclitaxel was not significantly associated with a negative outcome [250].
A secondary analysis of the IPFSG cohort (n = 1,600 patients) showed a 10-15% improvement in OS in all prognostic subgroups when treated with high-dose salvage therapy compared to standard dose therapy. This is being evaluated in an RCT of HDCT vs. conventional dose chemotherapy in patients with first-line relapse, which is currently underway (Tiger trial). When HDCT is used as a salvage treatment, sequential treatment cycles of high-dose carboplatin and etoposide (HD-CE) should be preferred to a single high-dose regimen as the former is associated with less toxicity-related deaths [245]. A recent SR confirmed the superiority of using at least two high-dose cycles in the salvage setting over a single high-dose cycle [251]. It is clearly of the utmost importance that these rare patients with relapse are treated within clinical trials and at specialised centres.
Table 9: The International Prognostic Factors Study Group Score for seminoma and non-seminoma that relapse after cisplatin-based first-line chemotherapy [189]
Points | -1 | 0 | 1 | 2 | 3 |
Variable | |||||
Histology | Seminoma | Non-seminoma | |||
Primary site | Gonadal | Retroperitoneal | Mediastinal | ||
Response | CR/PRm- | PRm+/SD | PD | ||
PFI | > 3 months | < 3 months | |||
AFP salvage | Normal | < 1000 | 1000 | ||
hCG salvage | < 1000 | 1000 | |||
LBB | No | Yes | |||
AFP = alpha-fetoprotein; CR = complete remission; PRm- = partial remission, negative markers;PRm+ = partial remission, positive markers; hCG = human chorionic gonadotrophin; LBB = liver, bone, brain metastases; PD = progressive disease; PFI = progression-free interval; SD = stable disease.
Table 10: Progression-free survival and overall survival estimates for all patients according to IGCCCG prognostic score for seminoma and non-seminoma that relapse after cisplatin-based first-line chemotherapy [190]
Score (n = 1,435) | N | % | HR | 2-years PFS (%) | 3-year OS (%) |
Very Low | 76 | 5.30 | 1 | 75.1 | 77.0 |
Low | 257 | 17.9 | 2.07 | 52.6 | 69.0 |
Intermediate | 646 | 45.0 | 2.88 | 42.8 | 57.3 |
High | 351 | 24.5 | 4.81 | 26.4 | 31.7 |
Very High | 105 | 7.3 | 8.95 | 11.5 | 14.7 |
Missing | 159 | - | - | - | - |
HR = hazard ratio; PFS = progression-free survival; n = number of patients; OS = overall survival.
7.3.5. Second relapse
No RCTs have been reported for patients with second relapse and overall conventional therapy does not appear effective. For patients who have received two series of conventionally dosed therapy (first line and first-salvage), HDCT with autologous stem cell support should be used [246]. With this the prospect of cure is only 20-25%.
Patients relapsing within four to eight weeks after platinum-based therapy, or who are progressing despite platinum-based therapy, as well as those relapsing shortly after HDCT, are considered as cisplatin refractory. Combinations of gemcitabine and oxaliplatin or the triple combination of gemcitabine, oxaliplatin and paclitaxel have resulted in response rates of 25-45% in this setting. Cisplatin re-challenge in association with gemcitabine and paclitaxel may be considered in patients with adequate renal function [252]. For patients with a second relapse not responding to the combination of oxaliplatin and gemcitabine or the triple combination, inclusion in clinical trials is encouraged.
Patients with a good response undergoing subsequent resection of residual tumour lesions may still have a 15-20% chance of long-term cure [237,253].
Various targeted agents have generally failed in refractory disease, including immune checkpoint inhibitors [245-251,254]. Trials combining PD1/PDL-1 and CTLA4 inhibitors are ongoing; however, even for those combinations early results are not encouraging.
7.3.5.1. Late relapse (more than two years after end of first-line treatment)
Late relapse is defined as recurrence more than two years after completion of successful primary treatment of metastatic TC [203]. According to a pooled analysis, this occurs in 1.4% and 3.2% of seminoma and non-seminoma patients, respectively [255].
Based on a population-based study all late-relapsing seminoma patients have viable GCT [256]. These can be treated with chemotherapy and radiotherapy.
In contrast, patients with late-relapsing NSGCT should undergo surgical resection when feasible, alone or in combination with chemotherapy. Some patients, including those with rapidly rising β-hCG, may benefit from induction salvage chemotherapy with subsequent reconsideration of surgery for resection of persisting residual masses [163]. In general, however, surgery represents the mainstay of treatment and it should be performed in most patients when feasible irrespective of the level of their tumour markers, in order to completely resect all viable GCT post-pubertal teratoma or TSTM [163,257]. Survival strongly relates to the histology of the recurrent lesions rather than that of the initial disease. If not completely resectable, biopsies should be obtained for histological evaluation to direct salvage chemotherapy based on the tumour phenotype. Review by an experienced pathologist is critical to avoid misinterpretation of the therapeutic morphological changes that occur with the treatment of GCT [258]. If the patient responds to salvage chemotherapy, secondary surgery should then be undertaken if feasible. With unresectable, but localised refractory disease, stereotactic or conventional radiotherapy may be considered. To avoid excess mortality, late relapses should be treated only at centres experienced in managing such patients [259].
7.3.6. Treatment of brain metastases
Brain metastases occur in the context of initial metastatic disease, systemic relapse and rarely as an isolated site of relapse. Long-term survival of patients presenting with brain metastases at diagnosis is poor (30- 50%) and even poorer when a site of recurrent disease (five-year survival-rate is 2-5%) [260,261]. A large international database comprising 523 patients reported 48% three-year OS rates in patients with brain metastases at initial diagnosis and 27% three-year OS rates for patients with brain metastases at relapse [47].
Chemotherapy as initial treatment proved effective in a first-line setting (potentially even as dose-intensified therapy upfront) with data also supporting the use of multimodal treatment particularly in relapsed disease [47]. Consolidation RT, even with total response after chemotherapy, should therefore be used in patients with brain metastases at relapse, but must be carefully discussed in a first-line setting [262]. Surgery may be considered in cases with a persistent solitary metastasis, depending on the systemic disease status, histology of the primary tumour and the location of the metastasis.
7.3.6.1. Guidelines for the treatment of metastatic testicular germ cell tumours
Summary of evidence | LE |
In the NSGCT good-prognosis-risk group (IGCCG), BEP x 3 is superior to other chemotherapy regimens. Toxicity is lower when treatment is delivered in five-day regimes rather than three-day regimes. | 1b |
In the NSGCT intermediate-prognosis-risk group (IGCCCG) BEP x 4 is the standard treatment of choice with a five-year survival of 89% in contemporary series. | 1b |
In pathological stage II NSGCT disease, RPLND performed in specialised centres without adjuvant chemotherapy results in 73-81% of long-lasting remissions. | 2b |
In patients with a poor-prognosis metastatic NSGCT (defined by IGCCCG), treatment with BEP x 4, results in a five-year PFS of 67%. There is no advantage in OS for high-dose chemotherapy. | 1b |
Patients with a poor-prognosis metastatic NSGCT and early unfavourable tumour marker decline may benefit from intensification of treatment with dose-dense chemotherapy, with improvement of PFS despite no benefit being observed for OS. | 1b |
Following first-line BEP chemotherapy, 6-10% of NSGCT residual masses contain active cancer, 50% have post-pubertal teratoma, and 40% comprise of necrotic-fibrotic tissue only. Figures regarding persistence of residual active are slightly lower in post chemotherapy residual masses < 1 cm. Currently there is no accurate prognostication method of histology. | 2b |
In CS IIA/B seminoma radiotherapy and chemotherapy treatment show similar effectiveness, with a non-significant trend towards greater efficacy of chemotherapy in CS IIB. However, risk of second malignancies and cardiovascular events is higher after radiotherapy. | 2a |
In metastatic seminoma stage > IIC, primary chemotherapy with BEP, tailored to the IGCCCG risk group, has proven superior to Carboplatin based chemotherapy. | 1b |
Fluorodeoxyglucose-positron emission tomography has a high NPV in patients with post-chemotherapy seminoma residual masses (> 3 cm) when performed more than two months after chemotherapy. | 2b |
Recommendations | Strength rating |
Treat low-volume non-seminomatous germ cell tumour (NSGCT) stage IIA/B with elevated markers like metastatic good- or intermediate-prognosis risk group IGCCCG with three or four cycles of cisplatin, etoposide, bleomycin (BEP). | Strong |
Nerve-sparing retroperitoneal lymph node dissection when performed by an experienced surgeon in a specialised centre is the recommended initial treatment in clinical stage (CS) IIA NSGCT disease without elevated tumour markers. | Weak |
Repeat staging after six weeks before making a final decision on further management should be considered in patients with small volume (CS IIA < 2 cm) marker-negative NSGCT. | Weak |
Treat metastatic NSGCT (stage > IIC) with an intermediate prognosis with four cycles of standard BEP. | Strong |
In metastatic NSGCT with a poor-prognosis, treat with one cycle of BEP, (or cisplatin, etoposide and ifosfamide [VIP], in cases with pulmonary dysfunction), followed by tumour marker assessment after three weeks. Continue the same schedule up to a total of four cycles with favourable marker decline. With unfavourable decline, initiate chemotherapy intensification. | Weak |
Perform surgical resection of visible (> 1 cm) residual masses after chemotherapy for NSGCT when serum levels of tumour markers are normal or normalising. | Strong |
Initially offer cisplatin chemotherapy according to IGCCCG prognosis groups, or alternatively radiotherapy to seminoma patients with stage II A/B and, inform the patient of potential long-term side effects of both treatment options. | Weak |
Treat seminoma stage IIC and higher, with primary chemotherapy according to IGCCCG classification (BEP x 3 in good-prognosis and BEP x 4 in intermediate prognosis). | Strong |