3. THE GUIDELINE
3.1. Phimosis and other abnormalities of the penile skin
The prepuce or foreskin of the penis is often a cause of concern to parents of young boys and physicians alike [13] with 10% seeking medical advice [14]. While there are some pathological abnormalities of the foreskin, these are in fact quite rare and must be discerned from physiological variations or developmental stages. In this chapter we highlight normal development, it’s variations and how to discern this from abnormal foreskin requiring treatment, as well as various treatment options.
3.1.1. Terminology, epidemiology and pathophysiology
At birth the foreskin can be retracted in 4% of boys. In 42% of neonates the tip of the glans cannot be visualised. By the end of the first year of life, retraction of the foreskin behind the glandular corona is possible in approximately 50% of boys; this increases to 89% by the age of three years. Non-retractability of the foreskin can be a physiological phase which does not require treatment in the absence of symptoms, such as painful erections or balanitis.
Phimosis
In phimosis the inability to retract the foreskin over the glans penis is due to a narrow ring in the prepuce. Several factors have been suggested to aid in the gradual dilation of this ring: histological changes in the prepuce, hormonal factors and stretching due to erections. While erections occur even antenatally, these may be insufficient to stretch the foreskin if it is relatively long, and therefore relative phimosis can be present for a prolonged period [15].
Epidemiological studies of the natural course of phimosis are difficult, as they are affected by treatment of a subgroup of subjects. Nonetheless, the incidence of phimosis is 9-20% in 5-13 year-olds and just 1% in males aged 16-18 years [15,16].
Preputial adhesions
Another cause of non-retractability of the prepuce are adhesions of the foreskin to the glans, and this must be distinguished from phimosis. Usually when adhesions are present, partial retraction is possible and the meatus can be visualised [16]. Adhesions are a physiological phenomenon of variable duration, present in 63% of 6-7 year-olds and 3% of 16-17 year-olds without phimosis [16]. Progressive separation of the inner prepuce from the glans is associated with build-up of epithelial debris (smegma) and aided by penile erections. During this process smegma can accumulate into nodules that may be mistaken for cysts. When released from between the skin layers smegma can resemble purulent discharge, especially when mixed with urine. There may temporarily be focal erythema. In the absence of other signs of infection, this should not be confused with balanitis.
Once adhesions between the glans and inner prepuce are resolved there may be ballooning of the foreskin during voiding, particularly if the opening of the prepuce is still relatively narrow. Ballooning is not a sign of obstructed voiding and uroflows have been shown to be normal with ballooning [17]. Therefore, ballooning may be a physiological phase, and it should only be considered a problem in case of (recurring) balanitis.
Paraphimosis
In paraphimosis the foreskin has been retracted and cannot be brought back down to cover the glans of the penis. In children it is most likely due to manipulation, with an incidence reported to be as low as 0.2% [14]. The risk of paraphimosis is higher if there is relative phimosis. The narrow ring in the retracted prepuce may constrict the shaft at the level of the sulcus, leading to edema of the glans and retracted foreskin. Impaired perfusion may lead to necrosis of the prepuce and ultimately of the glans. Paraphimosis must be regarded as a medical emergency requiring urgent treatment [18].
Balanitis/balanoposthitis
Balanoposthitis may be defined as erythema and swelling of the glans (balanitis) and/or foreskin (posthitis), with discharge of pus. It should not be confused with focal irritation due to retention of droplets of urine under the foreskin. Balanoposthitis may be seen in 6% of uncircumcised boys [14,19].
Balanitis xerotica obliterans
Balanitis xerotica obliterans (BXO) is a non-painful chronic inflammatory disease which may affect the glans, foreskin, meatus and urethra. As such it is a genital form of lichen sclerosus et atrophicus [15]. Balanitis xerotica obliterans may lead to scarring, phimosis and urethral outflow problems. Histological analysis of the prepuces of children and adolescents undergoing circumcision for medical reasons shows signs of BXO in 35%-53% [20]; in boys younger than ten years this is 17% [21,22].
Inconspicuous penis
There are several types of concealed or inconspicuous penis, which should be differentiated from truly small penis such as micropenis with abnormal size of the corporeal bodies or even aphallia.
Buried penis and megaprepuce are congenital anomalies in which the skin is folded abnormally around the shaft. The opening of the prepuce can be narrow, prohibiting retraction similar to regular phimosis, but may also be normal. Occasionally buried penis may be due to abnormal prepubic fat distribution, which may be self-limiting with growth or weight loss.
In webbed penis the penoscrotal angle is abnormal due to the scrotum being attached high on the ventral side of the shaft.
Trapped penis is an iatrogenic form of buried penis which may be caused by resection of too much skin during circumcision [23].
3.1.2. Classification and diagnostic evaluation
In order to determine which cases require treatment, phimosis should be divided into a physiological and pathological type. Physiological phimosis is most likely to resolve over time without intervention, whereas pathological phimosis may not.
In physiological phimosis there is no sign of scarring, and upon retraction the inner prepuce is seen bulging outward from the narrow ring in the prepuce (“pouting”). In pathological or secondary phimosis there is scarring, the narrow ring in the prepuce is fibrous, often white and thickened, and the inner layer of the prepuce is not seen coming out [24]. Balanitis xerotica obliterans is a special form of pathological phimosis.
The diagnosis of adhesions, phimosis and paraphimosis is made by physical examination alone, and this can differentiate between physiological variations or pathological abnormalities. If the prepuce is not retractable, or only partly retractable, and shows a constrictive ring upon retraction back over the glans penis, a disproportion between the width of the foreskin and the diameter of the glans penis has to be assumed. In addition to the constricted foreskin, the inner prepuce may be adherend to the glans and/or frenulum breve.
Balanitis xerotica obliterans remains a histopathological diagnosis as clinically discerning BXO from simple pathological phimosis by may be difficult, particularly to the untrained eye. Histopathological examination of resected foreskin is warranted due to the consequences of this diagnosis with regards to follow-up [25,26].
In buried penis, the shaft itself appears shorter upon inspection but is of normal size upon palpation, hence the name. In megaprepuce the shaft may have a normal appearance or it may resemble buried penis. The diagnosis is made based on the aspect of the penis during voiding. When the enlarged space between shaft and inner prepuce fills up with urine during voiding, this causes the entire penis to swell. Megaprepuce can be discerned from regular phimosis, in which only the tip of the penis may demonstrate ballooning. It may be helpful if caregivers show a photo or even video of the aspect of the penis during voiding.
3.1.3. Management
Hygiene
The foreskin should not be retracted for cleaning until this can be done easily. It should be stressed to parents/caregivers that forced retraction of a narrow foreskin may cause scar formation resulting in secondary pathological phimosis [27]. Care should be taken to reduce the foreskin back down over the glans to prevent paraphimosis. Once the foreskin is retractable this may be regularly done during bathing and becomes necessary for hygienic reasons from puberty. The production of smegma appears to increase at puberty, coinciding with the age at which most boys can retract their foreskin [24].
Conservative / medical management
Physiological phimosis and adhesions do not need treatment, unless there are accompanying urogenital abnormalities. Conservative medical treatment is a valid option for primary pathological phimosis. Class 4 corticosteroid therapies were more effective over placebo and manual stretching [28]. Topical corticoid (0.05-0.1%) can be administered twice a day over a period of 4-8 weeks with a success rate of > 80% [28-31]. A recent publication showed that lower class corticosteroids may be almost equally effective [32]. A recurrence rate of up to 17% can be expected [33]. Effectivity of topical corticosteroids is likely to be influenced by correct application, which must be directly onto the narrow ring under gentle retraction. Similarly, after finishing the corticosteroid treatment recurrence should be prevented by continuing daily retraction of the prepuce [34]. While all types of phimosis may respond to corticosteroid treatment, the success rate may be lower in pathological phimosis. If BXO is suspected, consultation with a dermatologist should be considered [35].
Corticosteroid treatment has no systemic side effects and mean blood cortisol levels are not significantly different from an untreated group of patients [36]. The hypothalamic pituitary-adrenal axis was not influenced by local corticoid treatment [37]. However, if treatment is continued for too long or too much product is used this may cause focal atrophy and vulnerability of the skin. In general, cream may be associated with dryness and irritation, due to the nature of the product compared to ointment. Adhesion of the foreskin to the glans does not respond to corticosteroid treatment [29].
Operative management
Circumcision for non-medical reasons, such as routine circumcision for cultural, religious or hygienic considerations, is not discussed in this chapter.
Medical indications for surgical intervention for phimosis are recurrent balanoposthitis or symptomatic therapy-resistant phimosis. Simple ballooning of the foreskin during micturition is not an indication for surgery per se. Several indications for circumcision in the absence of symptomatic phimosis have been proposed. In boys with increased risk of urinary tract infections (UTIs) due to congenital upper tract abnormalities, circumcision may be performed to reduce the risk of UTIs [38-41]. Male circumcision significantly reduces the bacterial colonisation of the glans penis with regard to both non-uropathogenic and uropathogenic bacteria [42]. However, resolution of phimosis by corticosteroid treatment may have similar results as it was also associated with substantial reduction in recurrent UTI in uncircumcised infants [43]. (See Chapter 3.9 on urinary tract infections in children and Chapter 3.14 on vesicoureteric reflux).
Routine neonatal circumcision to prevent penile carcinoma is not indicated. A meta-analysis could not find any risk in uncircumcised patients without a history of phimosis [44].
The type of operative treatment of phimosis in children is dependent on the caregivers’ preferences and can be preputioplasty or circumcision. In preputioplasty the objective is to preserve the prepuce while achieving a wider foreskin circumference with full retractability. Several surgical techniques have been described to achieve this goal: dorsal incision, partial circumcision, trident preputial plasty, combining two Z-plasties and Y-plasty [45,46]. The main disadvantage of preputioplasty is the inherent potential for recurrence of phimosis [47].
In circumcision, the prepuce is resected completely. Contra-indications for circumcision are: an acute local infection and congenital anomalies of the penis, particularly hypospadias, buried penis and megaprepuce, epispadias and congenital penile curvature, as the foreskin may be required for a reconstructive procedure [48,49].
When surgically correcting phimosis, additional issues should be addressed during the same session: adhesions are released, an associated frenulum breve is corrected by frenulotomy and the meatus is calibrated with meatoplasty added if necessary.
Paraphimosis treatment
Treatment of paraphimosis consists of manual compression of the oedematous tissue with a subsequent attempt to retract the tightened foreskin over the glans penis [50,51]. If this maneuver fails, a dorsal incision of the constrictive ring is required. Following acute redressing of the foreskin, additional treatment is recommended to correct any anomalies which increase the chance of recurrence. Patients should be counselled regarding prevention of paraphimosis by correctly redressing their foreskin after retraction.
3.1.4. Complications
Complications following circumcision vary and have been reported to be between 0-30% [52]. Hung et al., found 2.9% complications in non-neonates during a 5-year follow-up period; 2.2% were early (within 30 days after circumcision). Non-healing wounds, haemorrhage, wound infection, meatal stenosis, redundant skin, non-satisfying cosmetic appearance and trapped penis may all occur [53]. The incidence of post-circumcision meatal stenosis is higher in boys with confirmed BXO compared to those who underwent circumcision for phimosis without BXO (20% vs 6%) [25]. Overall, the risk of complications appears low when done by experienced practitioners in a medical setting.
3.1.5. Follow-up
Any preputial surgery requires early follow-up four to six weeks after surgery. In case of BXO, prolonged follow up is warranted and may involve a dermatologist. Balanitis xerotica obliterans is associated with meatal pathology (stenosis) after circumcision in up to 20% of boys [22,54,55].
3.1.6. Summary of evidence and recommendations for the management of phimosis
Summary of evidence | LE |
Non-retractability of the foreskin, preputial adhesions and ballooning may be a physiological phase before puberty and do not require treatment in the absence of symptoms. | 3 |
Forced retraction of a narrow foreskin should be avoided to prevent scar formation which may result in secondary pathological phimosis. | 3 |
Conservative treatment of phimosis with topical corticosteroids (ointment or cream) has a high success rate, but surgical treatment may be considered if preferred by caregivers or patients. | 1b |
Balanitis xerotica obliterans warrants prolonged follow up due to risk of meatal stenosis or urethral involvement. | 2 |
Recommendations | Strength rating |
Offer topical corticosteroids (ointment or cream) as first-line treatment in symptomatic phimosis. | Strong |
Consider surgical intervention if patient/caregivers prefer for symptomatic phimosis. | Strong |
Offer circumcision in case of Balanitis xerotica obliterans (BXO) or phimosis refractory to treatment. | Strong |
Offer treatment for asymptomatic phimosis in infants with a risk of recurrent urinary tract infection due to upper urinary tract abnormalities (vesicoureteral reflux or posterior urethral valves). | Strong |
Inform patients about the risk of meatal stenosis in BXO. | Strong |
Await spontaneous resolution of asymptomatic preputial adhesions before puberty. | Weak |
Treat paraphimosis by manual reposition and proceed to surgery if this fails. | Strong |
Do not perform simple circumcision if phimosis is associated with other penile anomalies such as buried penis, congenital penile curvature, epispadias or hypospadias. | Strong |
3.2. Management of undescended testes
3.2.1. Background
Cryptorchidism or undescended testis is one of the most common congenital malformations of male neonates. Incidence varies and depends on gestational age, affecting 1.0-4.6% of full-term and 1.1-45% of preterm neonates. Following spontaneous descent within the first months of life, nearly 1.0% of all full-term male infants still have undescended testes at one year of age [56]. This congenital malformation may affect both sides in up to 30% of cases [57]. In newborn cases with non-palpable or undescended testes on both sides and any sign of disorders of sex development (DSDs) like concomitant hypospadias, urgent endocrinological and genetic evaluation is required [58].
3.2.2. Classification
The term cryptorchidism is most often used synonymously for undescended testes. The most useful classification of undescended testes is distinguishing into palpable and non-palpable testes, and clinical management is decided by the location and presence of the testes (see Figure 1). Approximately 80% of all undescended testes are palpable [59]. Acquired undescended testes can be caused by entrapment after herniorrhaphy or spontaneously referred to as ascending testis.
Palpable testes include true undescended testes and ectopic testes. Non-palpable testes include intra-abdominal, inguinal, absent, and sometimes also some ectopic testes. Most importantly, the diagnosis of palpable or non-palpable testis needs to be confirmed once the child is under general anaesthesia, as this is the first step of any surgical procedure for undescended testes.
Figure 1: Classification of undescended testes
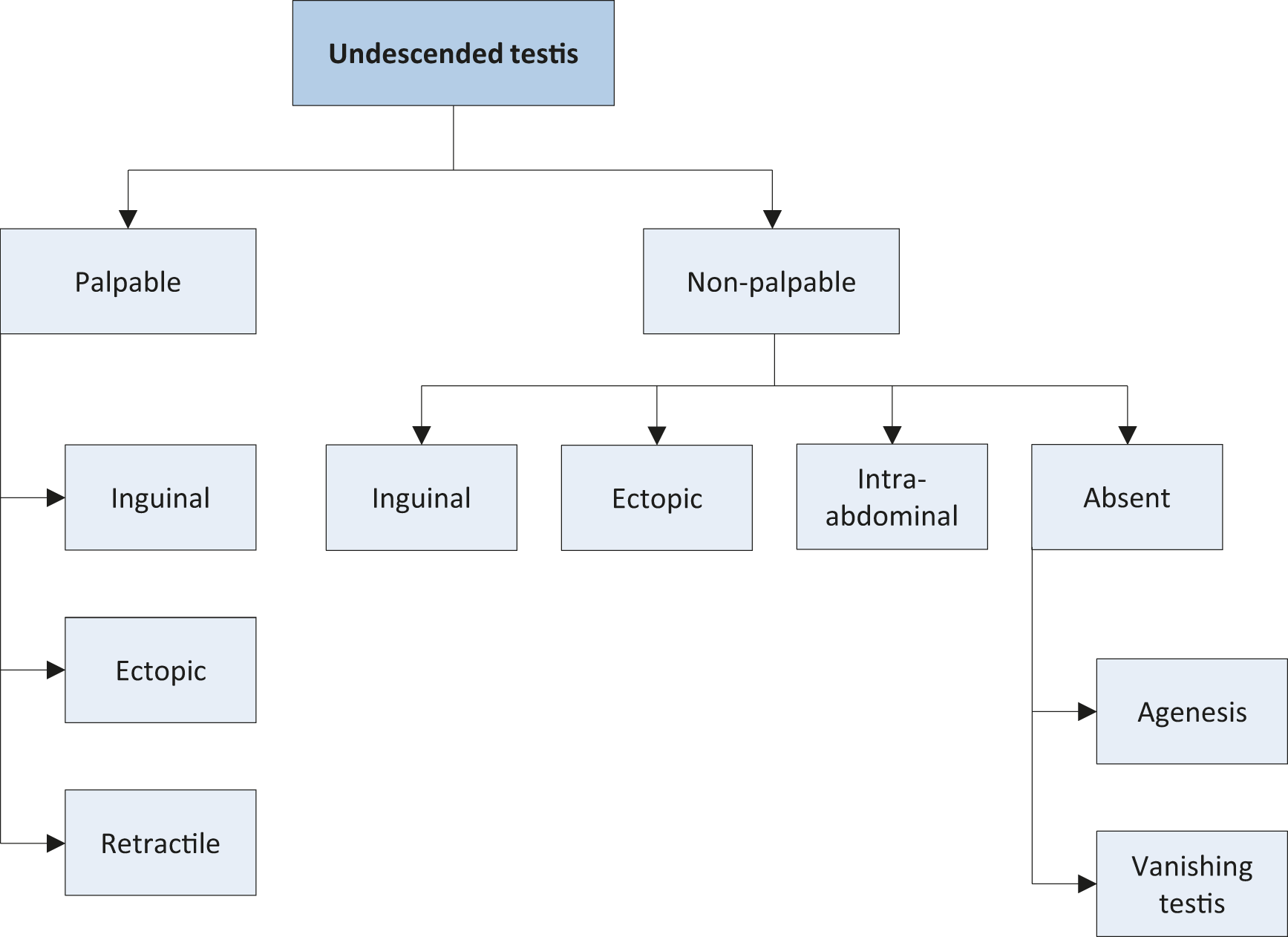
3.2.2.1. Palpable testes
Undescended testes
A true undescended testis is on its normal path of descent but is halted on its way down to the scrotum. Depending on the location, the testes may be palpable or not, as in the case of testes arrested in the inguinal canal.
Ectopic testes
If the position of a testis is outside its normal path of descent and outside the scrotum, the testis is considered to be ectopic. The most common aberrant position is in the superficial inguinal pouch. Sometimes an ectopic testis can be identified in a femoral, perineal, pubic, penile or even contralateral position. Usually, there is no possibility for an ectopic testis to descend spontaneously to the correct position; therefore, it requires surgical intervention. In addition, an ectopic testis might not be palpable due to its position.
Retractile testes
Retractile testes have completed their descent into a proper scrotal position but can be found again in a suprascrotal position along the path of their normal descent. This is due to an overactive cremasteric reflex [60]. Retractile testes can be easily manipulated down to the scrotum and remain there at least temporarily.
They are typically normal in size and consistency. However, they may not be normal and should be monitored carefully since up to one-third can ascend and become undescended [61].
3.2.2.2. Non-palpable testes
Among the 20% of non-palpable testes, 50-60% are intra-abdominal, canalicular or peeping (right inside the internal inguinal ring). The remaining 20% are absent and 30% are atrophic or rudimentary.
Intra-abdominal testes
Intra-abdominal testes can be located in different positions, with most of them being found close to the internal inguinal ring. However, possible locations include the kidney, anterior abdominal wall, and retrovesical space. In the case of an open internal inguinal ring, the testis may be peeping into the inguinal canal.
Absent testes
Monorchidism can be identified in up to 4% of boys with undescended testes, and anorchidism (bilateral absence) in < 1%. Possible pathogenic mechanisms include testicular agenesis and atrophy after intra-uterine torsion with the latter one most probably due to an in utero infarction of a normal testis by gonadal vessel torsion. The term “vanishing testis” is commonly used for this condition [62].
3.2.3. Diagnostic evaluation
History taking and physical examination are key in evaluating boys with undescended testes. Localisation studies using different imaging modalities are usually without any additional benefit.
3.2.3.1. History
Caregivers should be asked for maternal and paternal risk factors, including hormonal exposure and genetic or hormonal disorders. If the child has a history of previously descended testes this might be suggestive of testicular ascent [63]. Prior inguinal surgery is indicative of secondary undescended testes due to entrapment.
3.2.3.2. Physical examination
An undescended testis is pursued by carefully advancing the examining fingers along the inguinal canal towards the pubis region, perhaps with the help of lubricant. A possible inguinal testis can be felt to bounce under the fingers [64]. A non-palpable testis in the supine position may become palpable once the child is in a sitting or squatting position. If no testis can be identified along the normal path of descent, possible ectopic locations must be considered.
In the event of unilateral non-palpable testis, the contralateral testis needs to be examined. Its size and location can have important prognostic implications. Any compensatory hypertrophy suggests testicular absence or atrophy [65]. Nevertheless, this does not preclude surgical exploration since the sign of compensatory hypertrophy is not specific enough [66,67].
In the event of bilateral undescended testes and any evidence or sign of DSDs, such as genital ambiguity, or scrotal hyperpigmentation, further evaluation including endocrinological and genetic assessment becomes mandatory [68].
3.2.3.3. Imaging studies
Imaging studies cannot determine with certainty that a testis is present or not [69]. Ultrasound (US) lacks the diagnostic sensitivity to detect the testis confidently or establish the absence of an intra-abdominal testis [70]. Consequently, the use of different imaging modalities, such as US or magnetic resonance imaging (MRI) [71], for undescended testes is limited and only recommended in specific and selected clinical scenarios
(e.g., identification of Müllerian structures in cases with suspicion of DSDs) [70].
3.2.4. Management
Treatment should be started at the age of six months. After that age, undescended testes rarely descend [72].
Any kind of treatment leading to a scrotally positioned testis should be finished by twelve months, or eighteen months at the latest, because histological examination of undescended testes at that age has already revealed a progressive loss of germ cells and Leydig cells [73]. The early timing of treatment is also driven by the final adult results on spermatogenesis and hormone production, as well as on the risk of tumour development [74].
3.2.4.1. Medical therapy
Unfortunately, most of the studies on hormonal treatment have been of poor quality, with heterogeneous and mixed patient populations, testis location, schedules and dosages of hormonal administration. Additionally, long-term data are almost completely lacking.
Short-term side effects of hormonal treatment include increased scrotal erythema and pigmentation, and induction of pubic hair and penile growth. Some boys experience pain after intramuscular injection of human chorionic gonadotropin (hCG). All of these tend to regress after treatment cessation [75,76].
3.2.4.1.1. Medical therapy for testicular descent
Hormonal therapy using hCG or gonadotropin-releasing hormone (GnRH) is based on the hormonal dependence of testicular descent, but has a limited success rate of only 20% [77]. However, it must be taken into account that almost 20% of these descended testes have the risk of re-ascending later [78]. In general, success rates depend on testicular location. The higher the testis is located prior to therapy, the lower the success rate, suggesting that testicular position is an important determinant of success [75]. Some authors recommend combined hCG-GnRH treatment. Unfortunately, it is poorly documented and the treatment groups were diverse. Some studies reported successful descent in up to 38% of non-responders to monotherapy [79]. The Panel consensus is that endocrine treatment to achieve testicular descent is not recommended (LE: 4).
Human chorionic gonadotropin
Human chorionic gonadotropin stimulates endogenous testosterone production and is administered by intramuscular injection. Several dose and administration schedules are reported. There is no proven difference between 1.5 IU and weight-based doses up to 3.0 IU every other day for fourteen days [80]. Similar response rates were achieved with 500 IU once weekly and 1.5 IU three times weekly [81]. However, there is evidence that dosing frequency might affect testicular descent rates. Fewer lower dose injections per week for five weeks seem to be superior to one higher dose every seven to ten days for three weeks with regard to testicular descent [82].
Gonadotropin-releasing hormone
Gonadotropin-releasing hormone analogues (e.g., buserelin and gonadorelin) are available as nasal sprays, thus avoiding painful intramuscular injections. A typical dosage regimen consists of 1.2 mg per day in three divided doses, for four weeks. Success rates are wide ranging, from 9-60%, due to multiple treatment strategies and heterogeneous patient populations [83].
3.2.4.1.2. Medical therapy for fertility potential
Hormonal treatment may improve fertility indices [83,84] and therefore serve as an additional tool to orchidopexy. There is no difference in treatment with GnRH before (neo-adjuvant) or after (adjuvant) surgical orchidolysis and orchidopexy in terms of increasing fertility index, which may be a predictor for fertility later in life [85]. It is still unknown whether this effect on testicular histology persists into adulthood but it has been shown that men who were treated in childhood with buserelin had better semen analyses compared with men who had childhood orchidopexy alone or placebo treatment [83].
It is reported that hCG treatment may be harmful to future spermatogenesis through increased apoptosis of germ cells, including acute inflammatory changes in the testes and reduced testicular volume in adulthood [86].
Identification of specific subgroups of boys with undescended testes who would benefit from such an approach using hormones is difficult. Since these important data on specific groups as well as additional support on the long-term effects are still lacking, the Nordic consensus does not recommend hormonal therapy [87]. The consensus of the Panel is to recommend endocrine treatment with GnRH analogues in a dosage described above for boys with bilateral undescended testes to preserve fertility potential (LE: 4).
3.2.4.2. Surgical therapy
If a testis has not concluded its descent at the age of six months (corrected for gestational age), and since spontaneous testicular descent is unlikely to occur after that age, surgery should be performed within the subsequent year, and by age eighteen months at the latest [74]. In addition, early orchidopexy can be followed by partial catch-up testicular growth, which is not the case in delayed surgery [85]. All these findings recommend performing early orchidopexy between the ages of six and twelve months [72]. However, despite early and successful orchiopexy within the first year of life up to 25% of boys with non-syndromic undescended testes may be at risk of infertility based on hormonal and histological data, as a recently published series in 333 boys showed. This is especially true for bilateral cases; in addition, in about 5% of unilateral cases reduced numbers of germ cells were detected in testicular biopsies as well [88].
3.2.4.2.1. Palpable testes
Surgery for palpable testes includes orchidofunicolysis and orchidopexy, either via an inguinal or scrotal approach. The latter approach is mainly reserved for low-positioned, undescended testes, with the pros and cons of each method being weighed against each other [89].
3.2.4.2.1.1. Inguinal orchidopexy
Inguinal orchidopexy is a widely used technique with a high success rate of up to 92% [90]. Important steps include mobilisation of the testis and spermatic cord to the level of the internal inguinal ring, with dissection and division of all cremasteric fibres, to prevent secondary retraction and detachment of the gubernaculum testis. The patent processus vaginalis needs to be ligated proximally at the level of the internal ring, because an unidentified or inadequately repaired patent processus vaginalis is an important factor leading to failure of orchidopexy [91]. Any additional pathology has to be taken care of, such as removal of an appendix testis (hydatid of Morgagni). At this moment the size of the testis can be measured and the connection of the epididimis to the testis can be judged and described in the protocol. Some boys have a significant dissociation between testis and epididymis which is prognostically bad for fertility. Finally, the mobilised testicle needs to be placed in a sub-dartos pouch within the hemi-scrotum without any tension. If the length achieved using the above-mentioned technique is still inadequate, the Prentiss manoeuvre, which consists of dividing the inferior epigastric vessels and transposing the spermatic cord medially, in order to provide a straight course to the scrotum, might be an option [92]. With regard to fixation sutures, if required, they should be made between the tunica vaginalis and the dartos musculature [93]. Lymph drainage of a testis that has undergone surgery for orchidopexy may have changed from high retroperitoneal drainage to iliac and inguinal drainage, which might become important in the event of later malignancy [94].
3.2.4.2.1.2. Scrotal orchidopexy
Low-positioned, palpable undescended testis can be fixed through a scrotal incision including division of the gubernaculum, and the processus vaginalis needs to be probed to check for patency [95]. Otherwise, fixation in the scrotum is carried out correspondingly to the inguinal approach. In up to 20% of cases, an inguinal incision will be compulsory to correct an associated inguinal hernia [96]. Any testicular or epididymal appendages can be easily identified and removed. A systematic review has shown that the overall success rates ranged from 88-100%, with rates of recurrence and post-operative testicular atrophy or hypotrophy < 1% [89]. Another recently published systematic review and meta-analysis revealed similar outcome data regarding post-operative complications, including wound infection, testicular atrophy, testicular reascent, and hernia for palpable low positioned undescended testes. The only significant difference was the shorter operative time [97].
3.2.4.2.2. Non-palpable testes
For non-palpable testes, surgery must clearly determine whether a testis is present or not [98]. If a testis is found, the decision has to be made to remove it or bring it down to the scrotum. An important step in surgery is a thorough re-examination once the boy is under general anaesthesia, since a previously non-palpable testis might be identifiable and subsequently change the surgical approach to standard inguinal orchidopexy, as described above. Otherwise, the easiest and most accurate way to locate an intra-abdominal testis is diagnostic laparoscopy [99]. Subsequent removal or orchidolysis and orchidopexy can be carried out using the same approach to achieve the therapeutic aims [100]. Some tend to start with inguinal surgical exploration, with possible laparoscopy during the procedure [101]. If an ipsilateral scrotal nubbin is suspected, and contralateral compensatory testicular hypertrophy is present, a scrotal incision with removal of the nubbin, thus confirming the vanishing testis, is an option avoiding the need for laparoscopy [102].
During laparoscopy for non-palpable testes, possible anatomical findings include spermatic vessels entering the inguinal canal (40%), an intra-abdominal (40%) or peeping (10%) testis, or blind-ending spermatic vessels confirming vanishing testis (10%) [103].
If there is a vanishing testis, the procedure is finished once blind-ending spermatic vessels are clearly identified. If the vessels enter the inguinal canal, an atrophic testis may be found upon inguinal exploration or a healthy testis that needs to undergo standard orchidopexy [104]. A peeping testis can be placed down in the scrotum laparoscopically or via an inguinal incision [105]. Placement of an intra-abdominal testis can sometimes be a surgical challenge. Usually, testes lying > 2 cm above the internal inguinal ring may not reach the scrotum without division of the testicular vessels [106]. Under such circumstances, a Fowler-Stephens orchidopexy may be an option [107] (see Figure 2).
Proximal cutting and transection of the testicular vessels, with conservation of the collateral arterial blood supply, via the deferential artery and cremasteric vessels comprise the key features of the Fowler-Stephens procedure. Recently, a modification with low spermatic vessel ligation has gained popularity, allowing blood supply from the testicular artery to the deferential artery. An additional advantage is the position of the peritoneal incision, leading to a longer structure, to ease later scrotal placement [108]. Due to the nature of these approaches the testis is at risk of hypotrophy or atrophy if the collateral blood supply is insufficient [109]. The testicular survival rate in the one-stage Fowler-Stephens technique varies between 50 and 65% based on post-operative Doppler-ultrasound findings [110]. For two-stage procedures success rates increase up to 90% [111]. The advantages of two-stage orchidopexy, with the second part done usually six months after the first, are to allow for development of collateral blood supply and to create greater testicular mobility [112]. In addition, preservation of the gubernaculum may also decrease the chance of testicular atrophy [113]. An alternative might be microsurgical auto-transplantation, which has a success rate of up to 90%. However, this approach requires skilled and experienced surgeons and is performed in a limited number of centres [114].
3.2.4.2.3. Complications of surgical therapy
Surgical complications are usually uncommon, with testicular atrophy being the most serious. A systematic review revealed an overall atrophy rate for primary orchidopexy of 1.83%, 28.1% for one-stage Fowler-Stephens procedure, and 8.2% for the two-stage approach [115]. Other rare complications comprise testicular ascent and vas deferens injury besides local wound infection, dehiscence, and haematoma.
3.2.4.2.4. Surgical therapy for undescended testes after puberty
A study in 51 men diagnosed with inguinal unilateral undescended testis and a normal contralateral one, with no history of any previous therapy, demonstrated a wide range of changes upon histological evaluation. Nearly half of the study population still had significant germ cell activity at different maturation levels. Importantly, the incidence of intratubular germ cell neoplasia was 2% [116].
The Panel consensus recommends orchiectomy in post-pubertal boys with an undescended testis and a normal contralateral one in a scrotal position.
Figure 2: Treatment of unilateral non-palpable undescended testes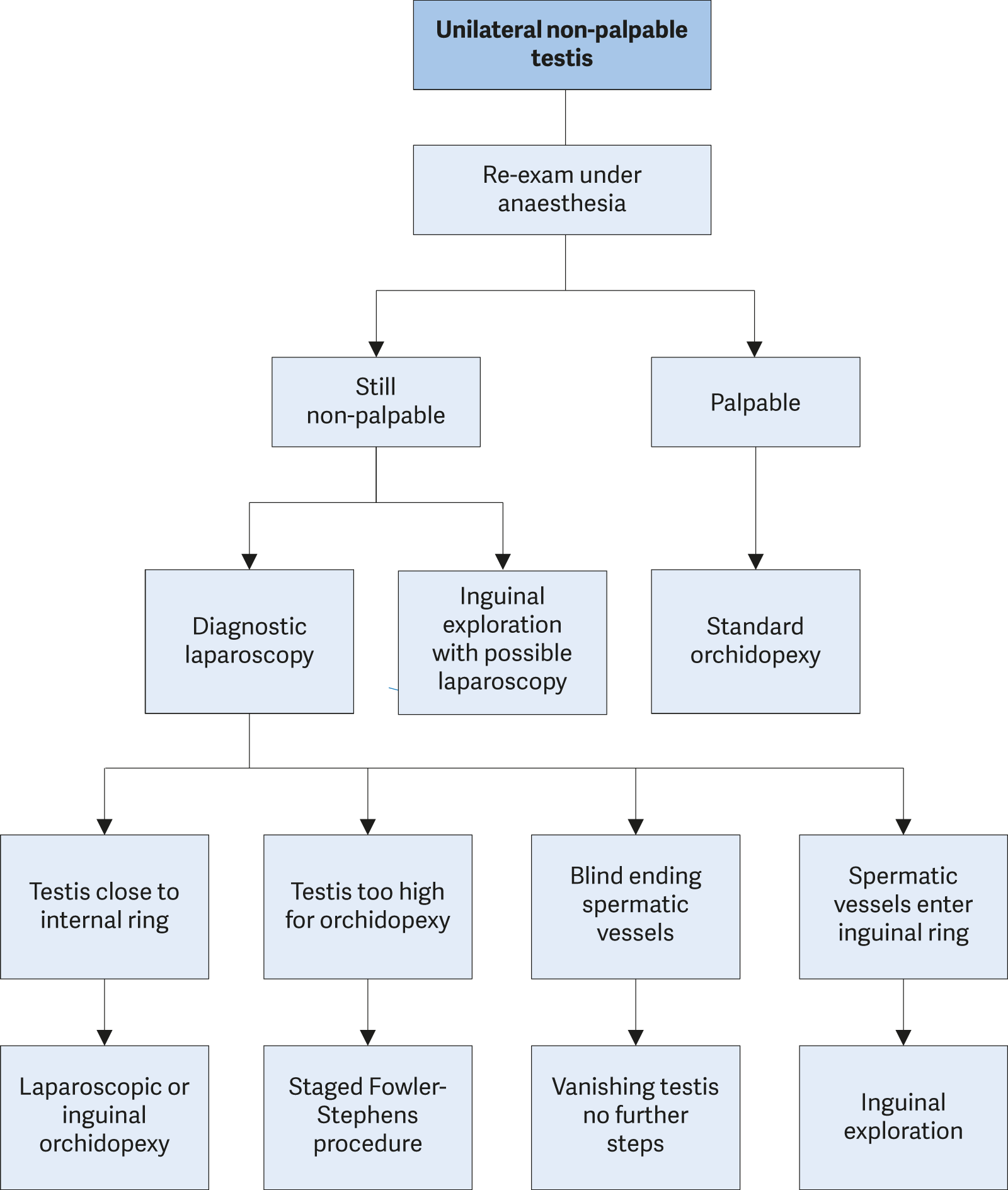
3.2.5. Undescended testes and fertility
The association of undescended testes with compromised fertility [117] is extensively discussed in the literature and seems to be a result of multiple factors, including germ cell loss, impaired germ cell maturation [118], Leydig cell diminution and testicular fibrosis [119].
Although boys with one undescended testis have a lower fertility rate, they have the same paternity rate as those with bilateral descended testes. Boys with bilateral undescended testes suffer both lower fertility and paternity rates. Fertility rate is the number of offspring born per mating pair, individual or population whereas paternity reflects the actual potential of fatherhood [120]. The age at which surgical intervention for an undescended testis occurs seems to be an important predictive factor for fertility later in life. Endocrinological studies revealed higher inhibin-B and lower follicle-stimulating hormone (FSH) levels in men who underwent orchidopexy at two years of age compared to individuals who had surgery later, which is indicative of a benefit of earlier orchidopexy [121]. In addition, others demonstrated a relation between undescended testes and increased loss of germ cells and Leydig cells, which is also suggestive of prompt orchidopexy being a significant factor for fertility preservation [122]. Outcome studies for untreated bilateral undescended testes revealed that 100% are oligospermic and 75% azoospermic. Among those successfully treated for bilateral undescended testes, 75% still remain oligospermic and 42% azoospermic [119].
In summary, early surgical correction of undescended testes is highly recommended before twelve months of age, and by eighteen months at the latest for preservation of fertility potential [73].
3.2.6. Undescended testes and malignancy
Boys who are treated for an undescended testis have an increased risk of developing testicular malignancy. Screening and self-examination both during and after puberty is therefore recommended [123]. A Swedish study, with a cohort of almost 17,000 men (56 developed a testicular tumour) who were treated surgically for undescended testes and followed for 210,000 person-years, showed that management of undescended testes before the onset of puberty decreased the risk of testicular cancer. The relative risk of testicular cancer among those who underwent orchidopexy before thirteen years of age was 2.2 compared to the Swedish general population; this increased to 5.4 for those treated after thirteen years of age [124].
A systematic review and meta-analysis of the literature have also concluded that pre-pubertal orchidopexy may reduce the risk of testicular cancer and that early surgical intervention is indicated in boys with undescended testes [125].
3.2.7. Summary of evidence and recommendations for the management of undescended testes
Summary of evidence | LE |
An undescended testis justifies treatment early in life to avoid loss of spermatogenic potential. | 2a |
A failed or delayed orchidopexy may increase the risk of testicular malignancy later in life. | 2a |
The earlier the treatment, the lower the risk of impaired fertility and testicular cancer. | 2a |
In unilateral undescended testis, fertility rate is reduced whereas paternity rate is not. | 1b |
In bilateral undescended testes, fertility and paternity rates are impaired. | 1b |
The treatment of choice for undescended testis is surgical replacement in the scrotum. | 1b |
The palpable testis is usually treated surgically using an inguinal approach. | 2b |
The non-palpable testis is most commonly approached laparoscopically. | 2b |
There is no consensus on the use of hormonal treatment. | 2b |
Recommendations | LE | Strength rating |
Do not offer medical or surgical treatment for retractile testes instead undertake close follow-up on a yearly basis until puberty. | 2a | Strong |
Perform surgical orchidolysis and orchidopexy before the age of twelve months, and by eighteen months at the latest. | 2b | Strong |
Evaluate male neonates with bilateral non-palpable testes for possible disorders of sex development. | 1b | Strong |
Perform a diagnostic laparoscopy to locate an intra-abdominal testicle. | 1a | Strong |
Hormonal therapy in unilateral undescended testes is of no benefit for future paternity. | 2a | Strong |
Offer endocrine treatment in case of bilateral undescended testes. | 4 | Weak |
Inform the patient/caregivers about the increased risk of a later malignancy with an undescended testis in a post-pubertal boy or older and discuss removal in case of a contralateral normal testis in a scrotal position. | 3 | Weak |
3.3. Testicular Tumours in prepubertal boys
3.3.1. Introduction
Testicular tumours account for approximately 1-2% of all paediatric solid tumours [126]. Testicular tumours in prepubertal boys differ in several aspects to testicular tumours in adolescent and adult men: they have a lower incidence, they have a different histologic distribution (teratomas and yolk sac tumours are more common and germ cell tumours are less common) and they are more often benign. A recent epidemiological study showed that in children under the age of 15 years the incidence is highest in Asia (4.2 per million) and South America (5 per million) and lowest in Europe (2.1 per million) and North America (2.5 per million). This is in contrast to the incidence in adolescent and young adults where the highest incidence is in Europe (137.4 per million), and North America (94.9 per million), while a lower incidence was observed in South and Central America (66.5 per million) and Asia (27.1 per million) [127]. For age distribution in prepubertal boys, there is a small peak around the age of two years [128]. Some recent studies demonstrated that up to 60-75% of the tumours are benign [126,129-133]. Intratubular neoplasia (TIN) is practically non-existent in children [134-137]. Testicular tumours can generally be classified as germ cell or stromal tumours. One specific tumour type is the gonadoblastoma, which contains germ cell and stromal cell tumour types and will occur almost exclusively in the setting of disorders of sexual differentiation [138].
In the past 30 years, it has clearly been shown, that there is a fundamental difference between testicular tumours in childhood and those in adulthood - not only in terms of the difference and incidence [127], but also in terms of histology [134]. In prepubertal boys, most intratesticular tumours are benign, whereas post puberty the tumours are most likely malignant.
3.3.2. Clinical presentation
Clinical presentation is a painless scrotal mass in more than 90% of the patients, detected by the caregiver, physician or the patient himself. A history of a trauma, pain or hernia is rare. A hydrocele can be found in
15-50% [130,139]. In boys with early onset of puberty (e.g. early penile and prepubic hair growth) as well as high testosterone and low gonadotropin levels, a Leydig cell tumour should be excluded [140].
In patients presenting with a scrotal mass, paratesticular tumours should also be taken into account as a differential diagnosis. However, these are even less common compared to intratesticular tumours. The spectrum of paratesticular tumours includes benign tumours such as leiomyoma, fibroma, lipoma, haemangioma, cystic lymphangioma and lipoblastoma as well as malignant tumours such as the paratesticular rhabdomyosarcoma with an excellent prognosis and the rare melanotic neuroectodermal tumour of infancy with a high recurrence rate [141-144]. As most of them are benign, intra-operative frozen section should be available during surgery. An organ sparing surgical approach is preferred in benign tumours, whereas in malignant tumour standard orchiectomy is carried out.
3.3.3. Evaluation
To confirm the diagnosis, a high-resolution US examination (7.5 – 12.5 MHz), preferably a doppler US, is required. The detection rate is almost 100% [145-148]. With high-resolution US, microlithiasis - small hyperdense areas without sound shadows - is increasingly seen in prepubertal boys. A recent meta-analysis showed that only 4 out of 296 boys (< 19 years of age diagnosed with microlithiasis) developed a testicular tumour of whom two previously had a testicular tumour on the opposite or ipsilateral site [149]. If microlithiasis shows up in patients with additional risk factors for testicular tumour, then the caregivers/patients should be informed about the increased risk and encouraged to carry out regular self-examinations - similar to patients treated for undescended testis [150]. There is no evidence, that regular sonographic follow-up is useful [149]. The risk for infertility may be higher in patients with microlithiasis and if these patients have any sign of infertility later, the risk of developing a tumour seems to be higher compared to patients without microlithiasis and infertility [151]. Due to the low incidence of a contralateral tumour, even in cases of testicular microlithiasis, there is no indication for contralateral testicular biopsy in prepubertal boys.
Age should be taken into account, when tumour markers are used. Human chorionic gonadotropin (ß-hCG) is derived from chorion carcinoma, embryonal carcinoma or seminoma. However, these tumours are extremely rare in prepubertal boys and therefore ß-hCG is not useful in prepubertal boys. Alpha-fetoprotein (AFP) has a clear limitation of its sensitivity and specificity in the first months of life [139] and sometimes takes up to twelve months before the serum concentration reaches the known standard values (< 10 ng/mL) [133,152]. It is produced by > 90% of yolk sac tumours. Teratomas can also produce AFP, but not to that extent of yolk sac tumours [153]. Alpha-fetoprotein should be measured before any therapeutic intervention (tumour enucleation/orchiectomy) and ideally should be available at the time of the procedure. Alpha-fetoprotein has a serum biological half-life of five days and should be measured five days after tumour resection/orchiectomy in those with an elevated AFP. There is no urgent need for pre-operative staging, as this has no consequence before the definitive histology is available.
3.3.4. Treatment/Management
If a testicular tumour is suspected, surgery with the option of intra-operative frozen section should be performed. It is not necessary to do this as an emergency procedure. However, in order to confirm the diagnosis and to avoid familial anxiety, the operation should be scheduled as soon as possible, preferably within the next few days. Organ-preserving surgery should be performed, whenever possible. A recent published review article showed that out of 227 patients with organ-sparing surgery only two cases (one in a patient with an epidermoid cyst and one in a patient with a mature teratoma) had a recurrence [154-156].
Orchiectomy could be considered only if normal testicular parenchyma is no longer detectable in the pre-operatively high-resolution US and/or the AFP is > 100 ng/mL in a > 12-month-old boy: highly suspicious of a yolk sac tumour.
For surgical technique, the Panel is in favour of an inguinal approach. Furthermore, clamping of the vessels has the advantage of a better view, when organ sparing surgery is performed. However, there is no evidence in the literature, that tumour-spread is prevented by clamping the vessels. Whenever possible, testis sparing surgery should be performed along with frozen sections during surgery to confirm the diagnosis (begin vs. malignant tumour) and to confirm if a microscopically margin-negative resection is performed, in which no gross or microscopic tumour remains in the primary tumour bed (R0 resection). In cases of an R0 resection, the tunica is closed and the testis is replaced in the scrotum. In case of R1 resection (removal of all macroscopic disease, but microscopic margins are positive for tumour) confirmed by frozen section in a malignant or potential malignant tumour, an orchiectomy should be performed at the same time of surgery. If the final pathology later demonstrates a R1 resection in a malignant tumour despite intra-operative negative margins on frozen section, an inguinal orchiectomy can safely be performed.
In patients with a malignant tumour (yolk sac tumour, immature teratoma) staging should be performed including an MRI of the abdomen and a CT-scan of the chest. If there is any suspicion of a non-organ confined tumour, the patient should be referred to a paediatric oncologist. In patients with the rare diagnosis of a Granulosa cell tumour, imaging of the abdomen to exclude enlarged lymph nodes is reasonable as this may be a potentially malignant tumour; in those with Sertoli or a Leydig cell tumour, an MRI is recommended, as 10% are malignant and the metastases do not respond very well to chemotherapy or radiation in the adult literature [157,158]. The TNM classification from 2015 for adult testicular tumours can be used in patients with a malignant tumour [159]. In benign tumours (mature teratoma, epidermoid cysts) no further staging is required.
3.3.5. Tumour entities in prepubertal boys
Teratomas are usually benign in prepubertal children and represent the greatest proportion of intratesticular tumours (around 40%) [126,160]. They present at a median age of 13 months (0-18 months). Only in adolescent and adults, they should be considered as malignant tumours. Histologically they can consist of a combination of the three primitive embryological germ-cell layers (ectoderm, mesoderm and endoderm). Most of these elements shows microscopically mature elements [161]; however, some immature teratomas in this age group have also been reported [162]. To exclude any malignant potential, like focal areas of a yolk-sac tumour, the entire specimen should be investigated. On US examination a heterogenous picture with some calcification is seen [163] and AFP should be less than 100 ng/mL in an infant. After organ-sparing surgery only one recurrence was reported in the literature [156].
Epidermoid cysts are of ectodermal origin and seem to be related to well-differentiated teratomas; they are always benign [161]. Keratin-producing epithelium is responsible for the keratinised-squamous-epithelial deposits, which appear hyperechogenic in an US [163]. Organ-sparing surgery should be performed and if confirmed by histology, there is no need for surveillance despite the fact that one “recurrence” has been reported thirteen years after diagnosis [155].
Juvenile granulosa cell tumours occur usually in the first year of life, typically within the first six months [164]. They are well circumscribed and have a typical yellow-tan appearance; 2/3 have cystic elements, 1/3 solid [164]. The stroma can be fibrous or fibromyxoid. So far, no recurrence has been reported after organ-sparing surgery [164,165].
Leydig cell tumours arising from the testosterone producing Leydig cells should be suspected in boys with early onset of puberty with high testosterone and low gonadotropin levels [140]. Patients are usually between six and ten years of age; the tumours are well circumscribed with yellow-brown nodules. In children there are no reports of malignant Leydig cell tumours and after organ sparing surgery, there are no reported recurrences to date [166,167]. In the adult literature, there is a malignancy rate of 10% reported and primary retroperitoneal lymphadenectomy should be discussed in cases with enlarged lymph nodes, as these metastases do not respond very well to chemotherapy or radiation [168].
Around 1/5 of the Sertoli-cell tumours occur in children; usually within the first year of life [169]. In the paediatric age group, the large-cell calcifying Sertoli cell tumours (LCCSCT) are the most common tumour variant [170,171]. They can occur in patients with complex dysplastic syndromes, such as the Carney or Peutz-Jeghers syndrome [171-173]. Except one case report with the histological diagnosis of a malignant LCCSCT [170], all other reported tumours are benign, therefore organ-sparing surgery should be performed.
Yolk sac tumours are the predominant prepubertal malignant germ cell tumours and may represent around 15% of the prepubertal tumours in boys [126]. They also have a number of other names: endodermal sinus tumours, juvenile embryonal carcinoma, clear cell carcinoma, orchioblastoma, vitellineum, archenteronoma and sometimes extraembryonal mesoblastoma [174]. They are histologically mostly solid, yellow-grey tumours. They occur usually within the first two years of life [175]. Up to 80-85% of the tumours are organ confined (Stage I) [176]. The tumour usually spreads haematogenously (chest). Twenty percent of those with Stage I disease may develop visible metastasis within the next two years. In a German study, 14 out of 91 patients with Stage I had a recurrence after observation – all were cured by chemotherapy alone. Four out of five with metastatic disease initially, were cured by chemotherapy after radical orchiectomy [177]. In a recent published series from China, 21 out of 90 paediatric patients with a Stage I yolk sac tumour received primary chemotherapy. One of the 21 had a recurrence, whereas 29 out of 69 who underwent surveillance after initial orchiectomy had a recurrence. The overall four-year survival rate was 97.8% [175], almost the same recurrence rate has also been reported by American oncology groups [178,179]. Therefore in patients with Stage I disease (no metastatic disease in the MRI-abdomen and CT scan of the chest as well as normal age-adapted AFP values) close follow-up together with the paediatric oncologists including AFP every two to three months and MRI of the abdomen is recommended, at least for the first two to three years [139]. This is especially recommended in those with invasions of the lymphatic vessels, as this has been shown to be a prognostic factor in a recent series [175]. In cases of recurrence, chemotherapy should be performed by paediatric oncologists according to national study protocols.
3.3.6. Follow-up
Regular US examination is recommended in the follow-up period to detect any recurrence and/or other abnormalities. As there are only a few studies with recurrence after testicular sparing surgery or orchiectomy, no clear recommendation can be made concerning the interval and the duration of follow-up. However, doing an US examination every three to six months within the first year seems reasonable, as few recurrences have been detected at this time and the rate of atrophy is extremely low after organ-sparing surgery [154]. Only in patients with a malignant tumour, regular follow-up examination after the first year of surgery seems reasonable (see above). The follow-up in patients with a Leydig cell tumour should include endocrinological examinations. Using the SEER data base, the five-year relative survival for testicular malignancies for patients < 14 years of age diagnosed with localised testicular cancer was 97.4%, and for those with distant disease 72.6% [180].
3.3.7. Congenital Adrenal Hyperplasia
Boys with a congenital adrenal hyperplasia (CAH) represent a special group. Up to a third of the patients have so-called testicular adrenal rest tumours (TARTs) This proportion increase with age [181,182]. It is most likely to be ectopic adrenal cells, which are growing under pathological stimulation from Adrenocorticotropic Hormone (ACTH) [183]. They have no malignant potential, but they can have a lasting impact on fertility by displacing the normal testicular parenchyma [183,184]. These patients should be offered US screening and advice on fertility with the option of cryopreservation [184]. As far as is known, no malignant tumour has been described in patients with a typical TART. As a result, the indication for surgical intervention in these patients to rule out a malignant tumour should be offered very cautiously.
Summary of evidence | LE |
Testicular tumours in prepubertal boys have a lower incidence and a different histologic distribution compared to the adolescent and adult patients. | 2a |
In prepupertal boys up to 60-75% of testicular tumours are benign. | 3 |
Recommendations | LE | Strength rating |
High-resolution ultrasound (7.5 – 12.5 MHz), preferably a doppler ultrasound, should be performed to confirm the diagnosis. | 3 | Strong |
Alpha-fetoprotein should be determined in prepubertal boys with a testicular tumour before surgery. | 2b | Strong |
Surgical exploration should be done with the option for frozen section, but not as an emergency operation. | 3 | Strong |
Organ-preserving surgery should be performed in all benign tumours. | 3 | Strong |
Staging (MRI abdomen/CT chest) should only be performed in patients with a malignant tumour to exclude metastases. | 3 | Strong |
Magnetic resonance imaging should only be performed in patients with potential malignant Leydig or Sertoli-cell-tumours to rule out lymph node enlargement. | 4 | Weak |
Patients with a non-organ confined tumour should be referred to paediatric oncologists post-operatively. | 4 | Weak |
3.4. Hydrocele
3.4.1. Epidemiology, aetiology and pathophysiology
Hydrocele is defined as a collection of fluid between the parietal and visceral layers of the tunica vaginalis [185]. Pathogenesis of primary hydrocele is based on patency of the processus vaginalis in contrast with secondary hydrocele. Incomplete obliteration of the processus vaginalis peritonei results in formation of various types of communicating hydrocele; a large open processus vaginalis allowing passage of abdominal viscera results in clinical hernia [186]. The exact time of spontaneous closure of the processus vaginalis is not known. It persists in approximately 80-94% of newborns and in 20% of adults [187]. If complete obliteration of the processus vaginalis occurs with patency of mid-portion, a hydrocele of the cord occurs. Scrotal hydroceles without associated patency of the processus vaginalis are also encountered in newborns [188]. Non-communicating hydroceles, based on an imbalance between the secretion and re-absorption of this fluid, are found secondary to minor trauma, testicular torsion, epididymitis, varicocele operation (due to ligation of the lymphatics) or may appear as a recurrence after primary repair of a communicating or non-communicating hydrocele.
3.4.2. Diagnostic evaluation
The classic description of a communicating hydrocele is that of a hydrocele that fluctuates in size, and is usually related to ambulation. It may be diagnosed by history-taking and physical investigation. Transillumination of the scrotum provides the diagnosis in the majority of cases, keeping in mind that fluid filled intestine and some pre-pubertal tumours may transilluminate as well [189,190]. If the diagnosis is that of a hydrocele, there will be no history of reducibility and no associated symptoms; the swelling is translucent, smooth and usually not tender. If there are any doubts about the character of an intrascrotal mass, scrotal US should be performed and has nearly 100% sensitivity in detecting intrascrotal lesions. Doppler US studies help to distinguish hydroceles from varicocele and testicular torsion, although these conditions may also be accompanied by a hydrocele.
3.4.3. Management
In the majority of infants, surgical treatment of hydrocele is not indicated within the first twelve months because of the tendency for spontaneous resolution [191] (LE: 2). Little risk is taken by initial observation as progression to hernia is rare and does not result in incarceration [191]. Early surgery is indicated if there is suspicion of a concomitant inguinal hernia or underlying testicular pathology [192,193] (LE: 2). Persistence of a simple scrotal hydrocele beyond twelve months of age may be an indication for surgical correction. There is no evidence that this type of hydrocele risks testicular damage. The natural history of hydrocele is poorly documented beyond the age of two years and according to a systematic review there is no good evidence to support current practice. Delaying surgery may reduce the number of procedures necessary without increasing morbidity [194].
The question of contralateral disease should be addressed by both history-taking and physical examination at the time of initial consultation [195] (LE: 2). In late-onset hydrocele, suggestive of a non-communicating hydrocele, there is a reasonable chance of spontaneous resolution (75%) and expectant management of six to nine months is recommended [196]. In the paediatric age group, the operation consists of ligation of the patent processus vaginalis or scrotal via inguinal incision and the distal stump is left open, whereas in hydrocele of the cord the cystic mass is excised or unroofed [190,192,197,198] (LE: 4). In expert hands, the incidence of testicular damage during hydrocele or inguinal hernia repair is very low (0.3%) (LE: 3). Laparoscopic hernia repair with percutaneous ligation of the patent processes vaginalis is a minimally invasive alternative to open inguinal herniorrhaphy [199,200]. Sclerosing agents should not be used because of the risk of chemical peritonitis in communicating processus vaginalis peritonei [190,192] (LE: 4). The scrotal approach (Lord or Jaboulay technique) is used in the treatment of a secondary non-communicating hydrocele.
3.4.4. Summary of evidence and recommendations for the management of hydrocele
Summary of evidence | LE |
In the majority of infants, surgical treatment of hydrocele is not indicated within the first twelve months due to the tendency for spontaneous resolution. Little risk is taken by initial observation as progression to hernia is rare. | 2a |
In the paediatric age group, an operation would generally involve ligation of the patent processus vaginalis via inguinal incision. | 4 |
Recommendations | LE | Strength rating |
In the majority of infants, observe hydrocele for twelve months prior to considering surgical treatment. | 2a | Strong |
Perform early surgery if there is suspicion of a concomitant inguinal hernia or underlying testicular pathology. | 2b | Strong |
Perform a scrotal ultrasound in case of doubt about the character of an intrascrotal mass. | 4 | Strong |
Do not use sclerosing agents because of the risk for chemical peritonitis. | 4 | Strong |
3.5. Acute scrotum
3.5.1. Epidemiology, aetiology and pathophysiology
Acute scrotum is a paediatric urological emergency, most commonly caused by torsion of the testis or appendix testis, or epididymitis/epididymo-orchitis [201-206]. Other causes of acute scrotal pain are idiopathic scrotal oedema, mumps orchitis, varicocele, scrotal haematoma, incarcerated hernia, appendicitis or systemic disease (e.g. Henoch-Schönlein purpura) [207-219]. Trauma can also be a cause of acute scrotum due to post-traumatic haematomas, testicular contusion, rupture, dislocation or torsion [220-225]. Scrotal fat necrosis has also been reported to be an uncommon cause of mild-to-moderate scrotal pain in pre-pubertal overweight boys after exposure to cold [226].
In this chapter testicular torsion and epididymitis are discussed, while recurrent epididymitis is discussed in the chapter dealing with infections. Torsion of the testis occurs most often in the neonatal period and around puberty, whereas torsion of the appendix testis occurs over a wider age range [227]. Epididymitis affects two age groups: less than one year and twelve to fifteen years [228,229]. One study predicted the annual incidence of epididymitis around 1.2 per 1,000 children [230].
Perinatal torsion of the testis most often occurs prenatally. Bilateral torsion comprises 11-21% of all perinatal cases [231]. Most cases of perinatal torsion are extravaginal, in contrast to the usual intravaginal torsion which occurs during puberty.
3.5.2. Diagnostic evaluation
Patients usually present with scrotal pain, except in neonatal torsion. The sudden onset of invalidating pain in combination with vomiting is typical for torsion of the testis or appendix testis [232,233].
In general, the duration of symptoms at presentation is shorter in testicular torsion (69% present within twelve hours) and torsion of the appendix testis (62%) compared to epididymitis (31%) [203,204,229]. Prepubertal males are more likely to present with atypical symptoms and delayed presentation and diagnosis, leading to delayed surgical intervention and a higher rate of orchiectomy, compared to postpubertal boys [234].
In the early phase, location of the pain can lead to diagnosis. Patients with acute epididymitis experience a tender epididymis, whereas patients with testicular torsion are more likely to have a tender testicle, in case of torsion of the appendix testis there may be isolated tenderness of the superior pole of the testis [229].
An abnormal (horizontal) position of the testis is more frequent in testicular torsion than epididymitis [203]. Looking for absence of the cremasteric reflex is a simple method with 100% sensitivity and 66% specificity for testicular torsion [228,233] (LE: 3). Elevation of the scrotum may reduce complaints in epididymitis, but not in testicular torsion.
Fever occurs more often in epididymitis (11-19%). The classical sign of a “blue dot” was found only in 10-23% of patients with torsion of the appendix testis [202,203,228,235]. In many cases, it is not easy to determine the cause of acute scrotum based on history and physical examination alone [201-206,228,235]. A positive urine culture is only found in a few patients with epididymitis [205,228,235,236]. It should be remembered that a normal urinalysis does not exclude epididymitis. Similarly, an abnormal urinalysis does not exclude testicular torsion.
Doppler US is useful to evaluate acute scrotum, with 63.6-100% sensitivity and 97-100% specificity, a positive predictive value of 100% and negative predictive value of 97.5% [237-242] (LE: 3). The use of Doppler US may reduce the number of patients with acute scrotum undergoing scrotal exploration, but it is operator-dependent and can be difficult to perform in pre-pubertal patients [239,243]. It may also show a misleading arterial flow in the early phases of torsion and in partial or intermittent torsion. Of key importance, persistent arterial flow does not exclude testicular torsion. In a multicentre study of 208 boys with torsion of the testis, 24% had normal or increased testicular vascularisation [239]. A comparison with the other side should always be done.
Better results were reported using high-resolution US (HRUS) for direct visualisation of the spermatic cord twist with a sensitivity of 97.3% and specificity of 99% [239,244] (LE: 2). A so-called positive whirlpool sign (the presence of a spiral-like pattern), has a pooled sensitivity and specificity of 0.73 (95% CI; 0.65-0.79) and 0.99 (95% CI; 0.92-0.99), respectively, and may be viewed as a definitive sign for testicular torsion. However, its role in neonates is limited [245].
Scintigraphy and, more recently, dynamic contrast-enhanced subtraction MRI of the scrotum also provide a comparable sensitivity and specificity to US [246-249]. These investigations may be used when diagnosis is less likely and if torsion of the testis still cannot be excluded from history and physical examination. This should be done without inordinate delays for emergency intervention [235].
The diagnosis of acute epididymitis in boys is mainly based on clinical judgement and adjunctive investigation. However, it should be remembered that findings of secondary inflammatory changes in the absence of evidence of an extra-testicular nodule by Doppler US might suggest an erroneous diagnosis of epididymitis in children with torsion of the appendix testes [250]. Pre-pubertal boys with acute epididymitis have an incidence of underlying urogenital anomalies of 25-27.6%. Complete urological evaluation in all children with acute epididymitis is still debatable [205,228,230].
3.5.3. Management
3.5.3.1. Epididymitis
In pre-pubertal boys, the aetiology is usually unclear, with an underlying pathology in about 25%. A urine culture is usually negative, and unlike in older boys, a sexually transmitted disease is very rare.
Antibiotic treatment, although often started, is not indicated in most cases unless urinalysis and urine culture show a bacterial infection [230,251]. Epididymitis is usually self-limiting and with supportive therapy (i.e., minimal physical activity and analgesics) heals without any sequelae (LE: 3). However, bacterial epididymitis can be complicated by abscess or necrotic testis and surgical exploration is required [252].
3.5.3.2. Testicular torsion
Manual detorsion of the testis is done without anaesthesia, and should be attempted in all patients if possible, because it is associated with improved surgical salvage rates [253]. It should initially be done by outward rotation of the testis - like opening a book -, unless the pain increases or if there is obvious resistance. Success is defined as the immediate relief of all symptoms and normal findings at physical examination [254] (LE: 3). Doppler US may be used for guidance [255]. Bilateral orchiopexy is still required after successful detorsion. This should not be done as an elective procedure, but rather immediately following detorsion. One study reported residual torsion during exploration in 17 out of 53 patients, including eleven patients who had reported pain relief after manual detorsion [254,256].
External cooling before exploration may be effective in reducing ischaemia reperfusion injury and preserving the viability of the torsed and the contralateral testis [257]. Medical treatments aimed at limiting such injury remain experimental [258-261].
Torsion of the appendix testis can be managed non-operatively with the use of anti-inflammatory analgesics (LE: 4). During the six-week follow-up, clinically and with US, no testicular atrophy was revealed. Surgical exploration is done in equivocal cases and in patients with persistent pain [242]. Although metachronous torsion of the appendix testis may occur in up to 8.5%, it is not necessary to explore the contralateral side, given the benign nature ot the problem. Besides it has been demonstrated that the NNT is 24 [262].
3.5.3.3. Surgical treatment
Testicular torsion is an urgent condition which requires prompt surgical treatment. The two most important determinants of early salvage rate of the testis are the time between onset of symptoms and detorsion, and the degree of cord twisting [263]. Severe testicular atrophy occurred after torsion for as little as four hours when the turn was > 360°. In cases of incomplete torsion (180-360°), with symptom duration up to twelve hours, no atrophy was observed. However, a necrotic or severely atrophied testis was found in all cases of torsion > 360° and symptom duration > 24 hours [264].
Early surgical intervention with detorsion (mean torsion time less than thirteen hours) was found to preserve fertility [265]. Urgent surgical exploration is mandatory in all cases of testicular torsion within
24 hours of symptom onset. In patients with testicular torsion > 24 hours, exploration may be performed as a semi-elective exploration procedure [263,264] (LE: 3), unless there is a clear history of torsion-detorsion in which urgent exploration should still be considered. In case of prolonged torsion (> 24 hours) it is still subject to debate whether the surgically detorsed testis should be preserved. An alternative to detorsion and fixation may be to perform orchiectomy. A study found that sperm quality was preserved after both orchiectomy and orchidopexy in comparison to normal control men, although orchiectomy resulted in better sperm morphology [266] Incision of the tunica albuginea with tunica vaginalis graft to prevent or treat compartment syndrome has also been suggested [267].
In neonates with signs of testicular torsion at birth the duration of symptoms will not be clear. The decision to perform surgical exploration should be weighed against the general condition of the child. In this age group the operation can safely be done under spinal anesthesia. New onset of symptoms of testicular torsion in neonates should be considered a surgical emergency similar to older boys.
During exploration, fixation of the contralateral testis is also performed. It is good clinical practice to also perform fixation of the contralateral testis in prenatal and neonatal torsion, although there is no literature to support this, and to remove an atrophied testicle [268]. Recurrence after orchidopexy is rare (4.5%) and may occur several years later. There is no consensus recommendation about the preferred type of fixation and suture material [269].
3.5.4. Follow-up
Patients require follow-up mainly for fertility issues and hormonal consequences. Despite timely and adequate detorsion and fixation of the testicle, up to half of the patients may develop testicular atrophy, even when
intra-operatively assessed as viable, and should be counselled accordingly [270,271].
3.5.4.1. Fertility
The results vary and are conflicting. In one study, unilateral torsion of the testis seriously intervened with subsequent spermatogenesis in about 50% of the patients and produced borderline impairment in another 20% [248]. Although, 30% of affected testicles with mumps orchitis show a degree of atrophy, long-term outcome in terms of fertility is not conclusive [272].
A recent study showed a normal pregnancy rate after unilateral testicular torsion, with no difference between the patients undergoing orchidopexy and those after orchidectomy [273].
3.5.4.2. Subfertility
Subfertility is found in 36-39% of patients after torsion. Semen analysis may be normal in only 5-50% in long-term follow-up [263]. Early surgical intervention (mean torsion time less than thirteen hours) with detorsion was found to preserve fertility, but a prolonged torsion period (mean 70 hours) followed by orchiectomy jeopardised fertility [265].
Subfertility and infertility are consequences of direct injury to the testis after the torsion. This is caused by the cut-off of blood supply, but also by post-ischaemia-reperfusion injury that is caused after the detorsion when oxygen-derived free radicals are rapidly circulated within the testicular parenchyma [263].
3.5.4.3. Androgen levels
Even though the levels of FSH, luteinising hormone (LH) and testosterone are higher in patients after testicular torsion compared to normal controls, endocrine testicular function remains in the normal range after testicular torsion [266].
3.5.4.4. Unanswered questions
Although testicular torsion is a common problem, the mechanism of neonatal and prenatal torsion is still not exactly known, as well as whether fixation of the contralateral testicle in these cases is really necessary. The influence of an atrophied testicle on fertility is also unclear.
Summary of evidence | LE |
Diagnosis of testicular torsion is based on presentation and physical exam. | - |
Doppler US is an effective imaging tool to evaluate acute scrotum and comparable to scintigraphy and dynamic contrast-enhanced subtraction MRI. | 2a |
Neonates with acute scrotum should be treated as surgical emergencies. | 3 |
Recommendations | Strength rating |
Testicular torsion is a paediatric urological emergency and requires immediate treatment. | Strong |
In neonates with testicular torsion perform orchidopexy of the contralateral testicle. In prenatal torsion the timing of surgery is usually dictated by clinical findings. | Weak |
Base the clinical decision on physical examination. The use of Doppler ultrasound to evaluate acute scrotum is useful, but this should not delay the intervention. | Strong |
Manage torsion of the appendix testis conservatively. Perform surgical exploration in equivocal cases and in patients with persistent pain. | Strong |
Perform urgent surgical exploration in all cases of testicular torsion within 24 hours of symptom onset. In prenatal torsion the timing of surgery is usually dictated by clinical findings. | Strong |
3.6. Hypospadias
3.6.1. Epidemiology, aetiology and pathophysiology
3.6.1.1. Epidemiology
The total prevalence of hypospadias in Europe is 18.6 new cases per 10,000 births (5.1-36.8) according to the recent EUROCAT registry-based study. This incidence was stable over the period of 2001 to 2010 [274,275]. The mean worldwide prevalence of hypospadias according to an extended systematic literature review varies: Europe 19.9 (range: 1-464), North America 34.2 (6-129.8), South America 5.2 (2.8-110), Asia 0.6-69, Africa 5.9 (1.9-110), and Australia 17.1-34.8. There are conflicting data on the recent trends of prevalence – different trends in Europe and an increasing trend in the USA [276,277].
3.6.2. Risk factors
Risk factors associated with hypospadias are likely to be genetic, placental and/or environmental [274,275] (LE: 2b). Interactions between genetic and environmental factors may help explain non-replication in genetic studies of hypospadias. Single nucleotide polymorphisms seemed to influence hypospadias risk only in exposed cases [275,278] (LE: 2b).
- An additional family member with hypospadias is found in 7% of families, but this is more predominant in anterior and middle forms [278-281].
- Endocrine disorders can be detected in rare cases.
- Babies with a low birth weight have a higher risk of hypospadias [278-281].
- Over the last 25 years, a significant increase in the incidence of hypospadias has been found.
- Endocrines disruptors are one component of a multi-factorial model for hypospadias.
- The use of oral contraceptives prior to pregnancy has not been associated with an increased risk of hypospadias in offspring, but their use after conception increased the risk of middle and posterior hypospadias [279-282] (LE: 2a).
3.6.3. Classification systems
Hypospadias are usually classified based on the anatomical location of the proximally displaced urethral orifice:
- distal-anterior hypospadias (located on the glans or distal shaft of the penis and the most commo type of hypospadias);
- intermediate-middle (penile);
- proximal-posterior (penoscrotal, scrotal, perineal).
The pathology may be different after skin release and should be reclassified accordingly. Anatomical location of the meatus may not always be enough to explain the severity and the complex nature of this pathology. Therefore, a simple classification related to severity of the problem, which considers penile length, glans size, shape, urethral plate quality and penile curvature is commonly used. In that classification there are two types: mild hypospadias (glanular or penile isolated hypospadias without associated chordee, micropenis or scrotal anomaly); and severe hypospadias (penoscrotal, perineal hypospadias with associated chordee and scrotal anomalies).
3.6.4. Diagnostic evaluation
Most hypospadias patients are easily diagnosed at birth (except for the megameatus intact prepuce variant which can only be seen after retraction of foreskin). Diagnosis includes a description of the local findings:
- position, shape and width of the orifice;
- presence of atretic urethra and division of corpus spongiosum;
- appearance of the preputial hood and scrotum;
- size of the penis;
- curvature of the penis on erection.
The diagnostic evaluation also includes an assessment of associated anomalies, which are:
- cryptorchidism (in up to 10% of cases of hypospadias);
- open processus vaginalis or inguinal hernia (in 9-15%).
Severe hypospadias with unilaterally or bilaterally impalpable testis, or with ambiguous genitalia, requires a complete genetic and endocrine work-up immediately after birth to exclude DSD, especially congenital adrenal hyperplasia. Urine trickling and ballooning of the urethra requires exclusion of meatal stenosis. The relationship between the severity of the hypospadias and associated anomalies of the upper or lower urinary tract are not confirmed [283] (LE: 3).
3.6.5. Management
3.6.5.1. Indication for reconstruction and therapeutic objectives
Differentiation between functionally necessary and aesthetically feasible operative procedures is important for therapeutic decision making.
The indications for surgery are:
- proximally located (ectopic) meatus causing ventrally deflected or spraying urinary stream;
- meatal stenosis;
- anterior curvature of the penis;
- cleft glans;
- rotated penis with abnormal cutaneous raphe;
- preputial hood;
- penoscrotal transposition;
- split scrotum.
Physical examination should check all anatomic components of the penis and evaluate the degree and nature of abnormality in each component. The examination should evaluate location of the meatus, the degree of proximal spongiosal hypoplasia, presence and degree of penile curvature, width and depth of the urethral plate, size of the glans, degree of ventral skin deficiency, availability of the foreskin and scrotal abnormalities like penoscrotal transposition and bifid scrotum.
As all surgical procedures carry the risk of complications, thorough pre-operative counselling of the caregiver is crucial.
To achieve an overall acceptable functional and cosmetic outcome, the penile curvature must be corrected and a neo-urethra of an adequate size with opening on the glans formed with proper skin coverage of the penile shaft [284] (LE: 4) (Figure 3). The use of magnifying spectacles and fine synthetic absorbable suture materials (6.0-7.0) are required. As in any penile surgery, exceptional prudence should be adopted with the use of cautery. Bipolar cautery is recommended. Knowledge of a variety of surgical reconstructive techniques, wound care and post-operative treatment are essential for a satisfactory outcome.
3.6.5.2. Pre-operative hormonal treatment
There is a lack of high-quality evidence to support that pre-operative hormonal treatment with androgen stimulation improves surgical outcomes. Yet, this treatment in the form of systemic testosterone, topical testosterone, and derivatives like dihydrotestosterone (DHT) and hCG are commonly being used to increase glans size pre-operatively to allow better tubularisation of the urethral plate and decrease the incidence of glans dehiscence. This treatment is usually limited to patients with proximal hypospadias, a small appearing penis, reduced glans circumference or reduced urethral plate [282,285,286]. Studies have shown that it leads to significant enlargement of the glans and shaft of the penis (LE: 1b) [287,288].
Moderate quality evidence from three randomised studies demonstrate significantly lower rates of urethracutaneous fistulae and re-operation rates in patients who received pre-operative hormonal treatment [289].
Pre-operative testosterone administration is most often well tolerated. Transient side effects on child´s behaviour, increased genital pigmentation, appearance of pubic hair, penile skin irritation and redness, increased erections and peri-operative bleeding have been reported, but no persistent side effects related to hormonal stimulation have been reported in the literature. There is also no evidence about possible effects on bone maturation [286,289,290].
There are concerns regarding the negative impacts of testosterone on wound-healing and increased bleeding during surgery. Cessation of therapy is recommended one or two months prior to surgery to avoid adverse effects during or after surgery [291].
3.6.5.3. Age at surgery
The age at surgery for primary hypospadias repair is usually 6-18 (24) months [284,292,293] (LE: 3). Age at surgery is not a risk factor for urethroplasty complication in pre-pubertal tubularised incised plate urethroplasty (TIP) repair [292] (LE: 2b). Complication rate after primary TIP repair was 2.5 times higher in adults than in the paediatric group according to a recent prospective controlled study [294] (LE: 2a).
3.6.5.4. Penile curvature
If present, penile curvature is often released by degloving the penis (skin chordee) and by excision of the connective tissue of the genuine chordee on the ventral aspect of the penis in up to 70% [295]. The urethral plate has well vascularised connective tissue and does not cause curvature in most cases [296,297]. The residual curvature is caused by corporeal disproportion and requires straightening of the penis, mostly using dorsal midline plication or orthoplasty (modification of the Nesbit plication with or without elevation of the neurovascular bundle). In more severe curvature (> 45°), which is often combined with a short urethral plate requiring transection, ventral penile lengthening is recommended to prevent shortening of the penis. This consists of a ventral transverse incision of the tunica albuginea extending from the 3 to 9 o´clock position patched with tunica vaginalis flap or graft, or in several short ventral corporotomies without grafting (LE: 2b) [298]. After the ventral lengthening, a shorter dorsal midline plication is usually added.
According to a retrospective study, dorsal plication remained significantly associated with recurrent ventral curvature independently of the other factors. Ventral corporeal grafting for severe penile curvature gives good long-term results and safety profiles for erectile function [299] (LE: 2b).
3.6.5.5. Urethral reconstruction
The mainstay of hypospadias repair is preservation of the well-vascularised urethral plate and its use for urethral reconstruction has become standard practice in hypospadias repair [297]. Mobilisation of the corpus spongiosum/urethral plate and the bulbar urethra decreases the need for urethral plate transection [298] (LE: 2b).
If the urethral plate is wide, it can be tubularised following the Thiersch-Duplay technique. If the plate is too narrow to be simply tubularised, it is recommended relaxing the plate by a midline incision and its subsequent tubularisation according to the Snodgrass-Orkiszewski TIP technique. This technique has become the treatment of choice in distal- and mid-penile hypospadias [300-303]. If the incision of the plate is deep, it is recommended to cover the raw surface with inner preputial (or buccal) inlay graft in primary and secondary repairs [304]. This also enables extension of the incision beyond the end of the plate to prevent meatal stenosis [305,306] (LE: 2a).
For distal forms of hypospadias, a range of other techniques is available (e.g. Mathieu, urethral advancement) [307] (LE: 2b). The TIP technique has become an option for proximal hypospadias as well [300-303,308]. However, urethral plate elevation and urethral mobilisation should not be combined with TIP repair because it results in focal devascularisation of the neo-urethra with symptomatic stricture development [309] (LE: 2b). The onlay technique using a preputial island flap is a standard repair, preferred in proximal hypospadias, if a plate is unhealthy or too narrow [295]. An onlay preputial graft is an option for single-stage repair [310] (LE: 2b).
If the continuity of the urethral plate cannot be preserved, single or two-stage repairs are used. For the former, a modification of the tubularised flap (Duckett tube), such as a tube-onlay or an inlay-onlay flap, or onlay flap on albuginea are used to prevent urethral stricture [311-313] (LE: 3); alternatively the Koyanagi-Hayashi technique is used [314-317]. The two-stage procedure has become preferable over the past few years because of lower recurrence of ventral curvature and more favourable results with variable long-term complication rates [306,311,318-322].
3.6.5.6. Re-do hypospadias repairs
For re-do hypospadias repairs, no definitive guidelines can be given. All the above-mentioned procedures are used in different ways and are often modified according to the individual findings and needs of the patient.
Figure 3: Algorithm for the management of hypospadias DSD = disorders of sex development; TIP = tubularised incised plate urethroplasty; MAGPI = meatal advancement and glanuloplasty incorporated.
DSD = disorders of sex development; TIP = tubularised incised plate urethroplasty; MAGPI = meatal advancement and glanuloplasty incorporated.
3.6.5.7. Penile reconstruction following formation of the neo-urethra
Following formation of the neo-urethra, the procedure is completed by glansplasty and by reconstruction of the penile skin. If there is a shortage of skin covering, the preputial double-face technique or placement of the suture line into the scrotum according to Cecil-Michalowski is used. In countries where circumcision is not routinely performed, preputial reconstruction can be considered. Preputial reconstruction carries a risk of specific complications but does not seem to increase the risk of urethroplasty complications [323]. In TIP repair, the use of a preputial dartos flap reduces the fistula rate [300,301] (LE: 2b).
3.6.5.8. Urine drainage and wound dressing
Urine is drained transurethrally (e.g. dripping stent) or with a suprapubic tube. No drainage after distal hypospadias repair is another option [324,325]. Circular dressing with slight compression, as well as prophylactic antibiotics during surgery, are established procedures [325] (LE: 4). Post-operative prophylaxis after hypospadias repair has limited benefit and it only reduces the risk of asymptomatic bacteriuria [326-328] (LE: 2b). There is no consensus on duration of stenting and dressing.
3.6.5.9. Outcome
Some studies have tried to determine risk factors for complications after hypospadias repair. An analysis of prospectively collected data found glans size (width < 14 mm), proximal meatal location and re-operation as independent risk factors for urethral complication [325,329]. Low surgeon volume independently increases the risk of fistula, stricture or diverticulum repair [325,330] (LE: 3).
A meta-analysis of complication rates of TIP repair found lower complication rates and incidence of re-operations in primary distal repairs (in 4.5%) than in primary proximal repairs (in 12.2%) and in secondary repair (in 23.3%) [300-303,307,325]. One should expect a predictable outcome with complication rates below 10% in distal hypospadias (fistula, meatal stenosis, dehiscence, recurrent ventral curvature, and haematoma) [330,331]. A similar incidence of fistula (3.4-3.6%) can be expected after the Mathieu and TIP repairs of distal hypospadias [308,332-334].
The complication rates of TIP and onlay repairs of primary severe hypospadias are similar, 24% and 27%, respectively. It is higher in free graft and in preputial island tube urethroplasty [295]. There is no strong evidence to suggest that the use of inlay grafts in TIP repair improves the outcome [335].
The complication rates of single-stage Koyanagi and Hayashi modification repairs go up 61%, according to a comparative study [314,325]. Staged buccal mucosa graft requires a redo grafting in 13% of patients, after the second stage more than one third of patients have complications, mostly with some degree of graft fibrosis [333,336]. A recent long-term study on two-stage flap repair showed a complication rate of 68% [325]; another study showed a re-operation rate of 28% [306,325].
3.6.6. Follow-up
Long-term follow-up is necessary up to adolescence to detect urethral stricture, voiding dysfunctions and recurrent penile curvature, diverticula, glanular dehiscence [337]. Up to half of complications requiring re-operation present after the first year post-operatively [338] (LE: 2b).
Obstructive flow curve is common after hypospadias repair and while most are not clinically significant, long-term follow-up is required [339-342] (LE: 2a). Urine flow is significantly lower in patients after hypospadias surgery, especially in those who had corrected chordee, but without significant association with lower urinary symptoms (LUTS) [343] (LE: 2a).
Objective scoring systems have been developed in order to evaluate the results of hypospadias surgery (HOSE) [344] (LE: 2b) and cosmetic appearance (HOPE-Hypospadias Objective Penile Evaluation) [345] (LE: 2a). The Pediatric Penile Perception Score (PPPS) is a reliable instrument to assess penile self-perception in children after hypospadias repair and for appraisal of the surgical result by caregivers and uninvolved urologists [346] (LE: 2a). Cosmetic results were judged more optimistically by surgeons as compared to caregivers using validated tools [347]. Current scoring systems have deficiencies in terms of patient reported outcomes, the long term outcomes and sexual function [348].
Adolescents and adults, who have undergone hypospadias repair in childhood, have a slightly higher rate of dissatisfaction with penile size, especially proximal hypospadias patients, but their sexual behaviour is not different from that of control groups [349,350] (LE: 2a-b). Another long-term follow-up of men born with hypospadias revealed, in a controlled study, that these patients are less satisfied with penile cosmetic outcome according to all parameters of the PPPS; there was a difference in penile length (9.7 vs. 11.6 cm) and more patients had lower maximum urinary flow. More prominent results were found in proximal hypospadias vs. controls [325,351].
According to a systematic review of long-term patient satisfaction with cosmetic outcomes [352]:
- patient perception of penile size does not differ greatly from the norm;
- patients approaching puberty have a more negative perception and are more critical about the cosmetic outcomes of surgery;
- patients report high levels of perception of deformity and social embarrassment.
There is a wide range of parameters that are measured to assess outcome after hypospadias surgery in the literature. There is a need for an age-specific core outcome set [353].
The majority of identified instruments focused on post-operative cosmetic satisfaction, with only one instrument considering urinary function, and no instruments evaluating sexual function and psychosocial sequelae [354].
3.6.7. Summary of evidence and recommendations for the management of hypospadias
Summary of evidence | LE |
The suggested age at surgery for primary hypospadias repair is 6-18 (24) months. | 3 |
The therapeutic objectives are to correct the penile curvature, to form a neo-urethra of an adequate size, to bring the new meatus to the tip of the glans, if possible, and to achieve an overall acceptable cosmetic appearance. | 4 |
Androgen stimulation therapy results in increased penile length and glans circumference. | 1b |
The complication rate is about 10% in distal and 25% in proximal hypospadias one-stage repairs. Higher and variable rates (between 28 and 68%) can occur in two-stage repairs. | 3 |
Sexual functions are usually well preserved but patients report high levels of perception of deformity and social embarrassment. | 2b |
Recommendations | Strength rating |
At birth, differentiate isolated hypospadias from disorders of sex development which are mostly associated with cryptorchidism or micropenis. | Strong |
Counsel caregivers on functional indications for surgery, aesthetically feasible operative procedures (psychological, cosmetic indications) and possible complications. | Strong |
In children diagnosed with proximal hypospadias and a small appearing penis, reduced glans circumference or reduced urethral plate, pre-operative hormonal androgen stimulation treatment is an option and the body of evidence to accentuate its harms and benefits is inadequate. | Weak |
For distal hypospadias, offer Duplay-Thiersch urethroplasty, original and modified tubularised incised plate urethroplasty; use the onlay urethroplasty or two-stage procedures in more severe hypospadias. A treatment algorithm is presented (Figure 3). Correct significant (> 30 degrees) curvature of the penis. | Weak |
Ensure long-term follow-up to detect urethral stricture, voiding dysfunctions and recurrent penile curvature, ejaculation disorder, and to evaluate patient´s satisfaction. | Strong |
Use validated objective scoring systems to assist in evaluating the functional and cosmetic outcome. | Strong |
3.7. Congenital penile curvature
3.7.1. Epidemiology, aetiology and pathophysiology
Congenital penile curvature presents penile bending of a normally formed penis due to corporal disproportion.
The incidence at birth is 0.6% and congenital penile curvature is caused by asymmetry of the cavernous bodies and an orthotopic meatus [355] because of developmental arrest during embryogenesis [356]. On the other hand, the incidence of clinically significant congenital penile curvature is much lower, because the extent of the curvature and its associated sexual dysfunction varies widely [357]. Most of the cases are ventral deviations (48%), followed by lateral (24%), dorsal (5%), and a combination of ventral and lateral (23%) [358]. Most ventral curvatures are associated with hypospadias due to chordee or ventral dysplasia of cavernous bodies [359]. Similarly, dorsal curvature is mostly associated with exstrophy/epispadias complex. Congenital penile curvature can decrease sexual quality of life in adults and successful repair can restore patients’ psychosocial and sexual wellbeing [360].
Curvature > 30° is considered clinically significant; curvature > 60° may interfere with satisfactory sexual intercourse in adulthood (LE: 4). Minor penile curvature may be the result of ventral penile skin deficiency only and should be distinguished from corporal anomalies. For penile curvature associated with hypospadias or epispadias refer to the relevant chapters.
3.7.2. Diagnostic evaluation
Penile curvature is frequently not documented until later in childhood since the penis only appears abnormal when erect. Patients are usually concerned with the aesthetic and/or functional aspects of their penis [361]. Besides exact history taking to exclude any possibility of acquired penile curvature (e.g., post-traumatic), a thorough clinical examination is mandatory. In addition, photo documentation of the erect penis clearly showing the curvature from different angles serves as a pre-requisite in pre-operative evaluation [362]. The exact degree of curvature is generally determined at the time of surgery using an artificial erection test.
3.7.3. Management
The treatment is surgical, starting with an artificial erection to determine the degree of curvature and to check symmetry after the repair [363]. The ultimate goal of any surgical method used to correct the curvature is to achieve corpora of similar size. Various procedures are in use ranging from simple de-gloving and plication procedures, to corporal rotation, use of free dermal or tunica vaginalis grafts, to complete penile disassembly techniques [364,365]. Reviews comparing the outcome of Nesbit/modified Nesbit procedures [366] to plication procedures [367] were able to demonstrate that while there is a decreased risk of complications and loss of sensation, it remains unclear whether plication techniques can lead to increased risk of recurrence [368,369]. Altogether these methods include the risk of post-operative shortening of the penis with an average loss of 2.5 cm in stretched penile length depending on the pre-operative degree of curvature and the type of repair used [370-372].
Recently the non-corporotomy technique has been introduced with promising results enabling correction of any degree of ventral curvature with neither shortening of the penis nor the risk of post-operative erectile dysfunction [373].
3.7.4. Summary of evidence and recommendations for the management of congenital penile curvature
Summary of evidence | LE |
Isolated congenital penile curvature is relatively uncommon. | 2a |
Congenital penile curvature is often associated with hypospadias. | 2a |
Diagnosis is usually made late in childhood. | 2a |
The penis only appears abnormal when erect. | 1b |
Congenital penile curvature can cause aesthetic as well as functional sexual problems. | 1b |
Congenital penile curvature is treated with surgery. | 1b |
The goal of surgery is to achieve corpora of similar size. | 1b |
Recommendations | LE | Strength rating |
Ensure that a thorough medical history is taken and a full clinical examination done to rule out associated anomalies in boys presenting with congenital curvature. | 1a | Strong |
Provide photo documentation of the erect penis from different angles as a prerequisite in the pre-operative evaluation. | 1b | Strong |
Perform surgery after weighing aesthetic as well as functional implications of the curvature. | 2b | Weak |
At the beginning as well as at the end of surgery, perform artificial erection tests. | 2a | Strong |
3.8. Varicocele in children and adolescents
3.8.1. Epidemiology, aetiology and pathophysiology
Varicocele is defined as an abnormal dilatation of testicular veins in the pampiniformis plexus caused by venous reflux. It is unusual in boys under ten years of age and becomes more frequent at the beginning of puberty. It is found in 14-20% of adolescents, with a similar incidence during adulthood. It appears mostly on the left side (78-93% of cases). Right-sided varicoceles are less common; they are usually noted only when bilateral varicoceles are present and seldom occur as an isolated finding [374-376].
Varicocele develops during accelerated body growth and increased blood flow to the testes, by a mechanism that is not clearly understood. Genetic factors may be present [377,378]. An anatomic abnormality leading to impaired venous drainage is expressed by the considerable prevalence of the left side condition where the internal spermatic vein drains into the renal vein. Varicocele can induce apoptotic pathways because of heat stress, androgen deprivation and accumulation of toxic materials [379,380]. In 70% of patients with grade II and III varicocele, left testicular volume loss was found. Abnormal reproductive hormonal levels (increased serum levels of FSH and LH, and decreased levels of inhibin B) and semen quality were reported in varicocele patients and were directly related to varicocele severity [381-383]. Severe histological damage is found in 20% of adolescents affected, with abnormal findings in 46% of affected adolescents. Histological findings are similar in children or adolescents and in infertile men. In about 20% of adolescents with varicocele, fertility problems will arise [384]. The adverse influence of varicocele increases with time.
3.8.2. Classification systems
Varicocele is classified into 3 grades [385]:
- Grade I - Valsalva positive (palpable at Valsalva manoeuvre only);
- Grade II - palpable (palpable without the Valsalva manoeuvre);
- Grade III - visible (visible at distance).
3.8.3. Diagnostic evaluation
Varicocele, being mostly asymptomatic, is generally noticed by the patient or caregivers, or discovered by the paediatrician at a routine visit. The diagnosis depends upon the clinical finding of a collection of dilated and tortuous veins in the upright posture; the veins are more pronounced when the patient performs the Valsalva manoeuvre. Clinical examination shoud include evaluation of the size of both testicles to detect a smaller testis.
In pre-pubertal boys and in isolated right varicocele, a renal US should be routinely added in order to rule out a secondary varicocele due to any retroperitoneal tumour extending into the renal vein and inferior vena cava.
Testicular volume is measured by US examination or by orchidometer. In adolescents, a testis that is smaller by > 2 mL or 20% compared to the other testis is considered to be hypotrophic [386]. Venous reflux into the plexus pampiniformis is diagnosed using Doppler US colour flow mapping in the supine and upright position and with valsalva manoeuvre [387]. Venous reflux detected on US only is classified as subclinical varicocele. Severity of reflux on Doppler US was shown to correlate with testicular damage [382].
Sperm analysis in principle allows assessment of testicular function, but the World Health Organization (WHO) parameters are not intended for pre-pubertal patients, and spontaneous improvements of abnormal sperm analyses has been observed in pre-pubertal patients [388]. Moreover, sperm analysis encounters cultural/ethical barriers in children [389]. Therefore, semen analysis in not widely used and it is generally recommended only in older adolescents.
In order to assess testicular injury in adolescents with varicocele, supranormal FSH and LH responses to the luteinising hormone-releasing hormone (LHRH) stimulation test are considered reliable, because histopathological testicular changes have been found in these patients [390,391].
3.8.4. Management
There is no evidence that treatment of varicocele at paediatric age will offer a better andrological outcome than an operation performed later and earlier diagnosis should not convey a more pressing need to intervene [392,393]. Beneficial effect of pubertal screening and treatment for varicocele regarding the preservation of fertility and final chance of paternity is controversial [394-396]. The recommended indication criteria for correction for varicocele in children and adolescents are [375]:
- Varicocele associated with a small testis (this should be confirmed on two subsequent visits performed six months apart) as asynchronous testicular growth can account for a temporary asymmetry also in a considerable number of healthy adolescents [397].
Additional scenarios where varicocele treatment can be considered on a case by case basis include:
- Symptomatic varicocele [396]. Pain is present in 2-10% of varicoceles. The association between varicocele and pain is unclear and patients should be informed that pain can persist after varicocelectomy in 20% of cases [398].
- Additional testicular condition affecting fertility such as a contralateral testicular condition.
- Bilateral palpable varicocele.
- Pathological sperm quality (in older adolescents).
- Cosmetic reasons related to their scrotal swelling.
A reduced total testicular volume (left + right) in comparison with normal testes is a promising indication criterion, once the normal values are available [383,392]. Repair of a large varicocele, causing physical or psychological discomfort, may also be considered. Other varicoceles should be followed-up until a reliable sperm analysis can be performed.
3.8.4.1. Surgical management
Surgical intervention is based on ligation or occlusion of the internal spermatic veins.
Ligation is performed at different levels:
- inguinal (or subinguinal) microsurgical ligation;
- suprainguinal ligation, using open or laparoscopic techniques [399-402].
The advantage of the former is the lower invasiveness of the procedure, while the advantage of the latter is a considerably lower number of veins to be ligated and safety of the incidental division of the internal spermatic artery at the suprainguinal level.
For surgical ligation, some form of optical magnification (microscopic or laparoscopic) should be used because the internal spermatic artery is 0.5 mm in diameter at the level of the internal ring [399,401]. In suprainguinal approach, an artery sparing varicocelectomy may not offer any advantage in regards to catch-up growth and is associated with a higher incidence of recurrent varicocele [403,404].
Lymphatic-sparing varicocelectomy is preferred to prevent hydrocele formation and testicular hypertrophy development and to achieve a better testicular function according to the LHRH stimulation test [399,400,405,406]. The methods of choice are subinguinal or inguinal microsurgical (microscopic) repairs, or suprainguinal open or laparoscopic lymphatic-sparing repairs [399,401,407,408]. In the later, intrascrotal/intratesticular injection of isosulphan blue was recommended to visualise the lymphatic vessels [409,410].
3.8.4.2. Radiological management
Angiographic occlusion of the internal spermatic veins also meets the requirements of lymphatic sparing repair. It is based on retrograde or antegrade sclerotisation of the internal spermatic veins [411,412]. However, although this method is less invasive and may not require general anaesthesia, it is associated with radiation burden, which is less controllable in the antegrade technique [375,411,412].
There is low to moderate level of evidence that radiological or surgical treatment of adolescent varicocele is associated with improved testicular size/growth and sperm concentration. Several authors reported testicular catch-up growth after varicocelectomy in adolescents [413,414]. An average proportion of catch-up growth of 76.4% (range: 52.6-93.8%) has been found according to a meta-analysis [415] (LE: 2a). However, this may partly be attributable to testicular oedema associated with the division of lymphatic vessels [405]. Improvement in sperm parameters has been demonstrated after adolescent varicocelectomy [390,416-418]. In one study, microsurgical varicocele repair in adolescents with varicocele significantly increased paternity rates and decreased time to conception post-operatively, but this needs to be confirmed in other series. The ultimate effects on fertility and paternity rates are not known [419].
The Panel conducted a systematic review and meta-analysis regarding the treatment of varicocele in children and adolescents [420]. Of the 1,550 articles identified, 98 articles including 16,130 patients were eligible for inclusion (12 RCTs, 47 NRSs and 39 case series). The key findings are summarised in the following paragraphs.
The meta-analysis of the twelve RCTs revealed that varicocele treatment improved testicular volume (mean difference 1.52 mL, 95% CI 0.73-2.31) and increased total sperm concentration (mean difference 25.54, 95% CI 12.84-38.25) when compared with observation. Lymphatic sparing surgery significantly decreased hydrocele rates (p=0.02) and the OR was 0.08 (95% CI 0.01, 0.67). Due to the lack of RCTs, it was not possible to identify a surgical technique as being superior to the others. It remains unclear whether open surgery or laparoscopy is more successful for varicocele treatment (OR ranged from 0.13 to 2.84).
The treatment success rates (disappearance of varicocele) were between 85.1% and 100% whereas the complication rates were between 0% and 29% in the included studies. The most common complication reported was hydrocele. Resolution of pain after treatment was more than 90% in the reported series.
The major reason for varicocele recurrence is the persistence of branched spermatic veins that were not ligated during the initial repair. Treatment of recurrence is warranted only in those patients with clinical recurrence that show no improvement in testicular asymmetry or remain symptomatic. Treatment of recurrence can be surgical or via embolisation. Generally, a technique different from the primary repair is recommended to operate in a virgin field [421].
In conclusion, moderate evidence exists on the benefits of varicocele treatment in children and adolescents in terms of testicular volume and sperm concentration. Current evidence does not demonstrate superiority of any of the surgical/interventional techniques regarding treatment success. Lymphatic sparing surgery significantly decreases hydrocele formation. Long-term outcomes, including paternity and fertility, still remain unknown.
3.8.5. Summary of evidence and recommendations for the management of varicocele
Summary of evidence | LE |
Varicocele becomes more frequent at the onset of puberty and is found in 14-20% of adolescents. Testicular problems are reported in up to 20% of patients, but the final effect on paternity is unknown. | 3 |
After adolescent varicocelectomy, testis catch-up growth and improvement in sperm parameters has been demonstrated. | 3 |
There is no evidence that treatment of varicocele at paediatric age will offer a better andrological outcome than an operation performed later. | 1a |
Division of testicular lymphatics leads to hydrocele in up to 40% and to testicular hypertrophy. Lymphatic sparing surgery significantly decrease hydrocele rates. | 2 |
Recommendations | Strength rating |
Examine varicocele in the standing position and classify into three grades. | Strong |
Use scrotal ultrasound to evaluate testicular volume and to detect venous reflux in the supine and upright position and during Valsalva maneuver. | Strong |
In all pre-pubertal boys with a varicocele and in all isolated right varicoceles, perform standard abdominal ultrasound to rule out a retroperitonal mass. | Strong |
Inform caregivers and patients and offer surgery for varicocele associated with a persistent small testis (size difference of > 2 mL or 20%). | Strong |
Varicocele treatment can be also considered under the following circumstances: symptomatic varicocele; additional testicular condition affecting fertility such as a contralateral testicular condition; bilateral palpable varicocele; pathological sperm quality (in older adolescents); cosmetic reasons related to their scrotal swelling. | Weak |
Use some form of optical magnification (microscopic or laparoscopic magnification) for surgical ligation. | Strong |
Use lymphatic-sparing varicocelectomy to prevent hydrocele formation. | Strong |
3.9. Urinary tract infections in children
3.9.1. Epidemiology, aetiology and pathophysiology
Urinary tract infections (UTIs) represent the most common bacterial infections in children [422-424]. There are several classification systems used to define a UTI. In neonates, the symptoms differ in many aspects from those in infants and children. The prevalence is higher; there is a male predominance; infections caused by other organisms than Escherichia coli are more frequent; and there is a higher risk of urosepsis [425,426].
In children presenting with urinary symptoms a pooled prevalence of UTI was 7.8% (CI: 6.6-8.9) [425]. The incidence varies depending on age and sex. One meta-analysis showed that in children presenting with fever in the first three months of life UTIs were present in 7.5% of girls, 2.4% (CI: 1.4-3.5) of circumcised boys, and 20.1% (CI: 16.8-23.4) of uncircumcised boys [425]. The incidence for boys is highest during the first six months of life (5.3%) and decreases with age to around 2% for the ages 0-6 years. In girls, UTIs are less common during the first six months of life (2%) and incidence increases with age to around 11% for the ages 0-6 years [427].
Associated risk factors for recurrent UTIs include bladder and bowel dysfunction (BBD), vesicoureteral reflux (VUR) and obesity [428-430]. In older children a delay in treatment is more often seen than in younger infants [431]. These risk factors in combination with delay in treatment have been associated with renal scarring [432]. Recurrent febrile UTIs, especially in combination with high-grade VUR, lead to renal scarring [433,434]. Each new febrile UTI increases the risk of renal scarring with an incidence of renal scarring after the first UTI, of 2.8% (CI:1.2-5.8), 25.7% (CI:12.5-43.3) after the second infection and up to 28.6%
(CI:8.4-58.1) after 3 or more febrile UTIs [434].
The leading causative organism for UTIs has been E. coli, but other bacteriae have been rising in prevalence. In a large European study E. coli was found in less than 50% of urine cultures. Klebsiella pneumoniae, Enterobacter spp., Enterococcus spp., Pseudomonas spp., Proteus spp. and Candida spp. are more frequent in nosocomial infections than in community-acquired UTIs, even though, their prevalence has increased outside of the hospital setting [435]. Neonatal UTI is frequently complicated by bacteraemia. In a retrospective study, 12.4% of blood cultures from neonates admitted for UTI were positive for bacteraemia [436], however, it is less frequent in community-acquired than in nosocomial UTI [436,437].
3.9.2. Classification systems
There are five widely used classification systems according to; site, severity, episode, symptoms and complicating factors. For acute treatment, site and severity are most important.
3.9.2.1. Classification according to site
Lower urinary tract infection (cystitis) is an inflammatory condition of the urinary bladder mucosa with general signs and symptoms including infection, dysuria, frequency, urgency, malodorous urine, enuresis, haematuria, and suprapubic pain.
Upper urinary tract infection (pyelonephritis) is a diffuse pyogenic infection of the renal pelvis and parenchyma. The onset of pyelonephritis is generally abrupt. Clinical signs and symptoms include fever
(> 38°C), chills, costovertebral angle or flank pain, and tenderness.
3.9.2.2. Classification according to severity
In a lower UTI, children may have only mild pyrexia; are able to take fluids and oral medication; are only slightly or not dehydrated; and have a good expected level of compliance. When a low level of compliance is expected, such children should be managed as those with severe UTI. In severe UTI, infection is related to the presence of fever of > 39°C, the feeling of being ill, persistent vomiting, and moderate or severe dehydration. Most severe UTIs are upper urinary tract infections.
3.9.2.3. Classification according to episode first/persistent/recurrent/breakthrough
The first UTI may be a sign of anatomical anomalies. Anatomical evaluation is recommended (see below). Recurrent infection can be divided into unresolved and persistent infection.
In unresolved infection, initial therapy is inadequate for elimination of bacterial growth in the urinary tract (inadequate therapy, inadequate antimicrobial urinary concentration [poor renal concentration/ gastrointestinal malabsorption], and infection involving multiple organisms with differing antimicrobial susceptibilities).
Persistent infection is caused by re-emergence of bacteria from a site within the urinary tract coming from a nidus for persistent infection that cannot be eradicated (e.g. infected stones, non-functioning or poorly functioning kidneys/renal segments, ureteral stumps after nephrectomy, necrotic papillae, urachal cyst, urethral diverticulum, peri-urethral gland, vesicointestinal, rectourethral or vesicovaginal fistulas). The same pathogen is identified in recurrent infections, but episodes of sterile urine may occur during and shortly following antimicrobial treatment.
A breakthough infection in patients under antibacterial prophylaxis is usually caused by resistent bacteria, parental non-compliance and/or severe urogenital anomalies [438,439].
In re-infection, each episode can be caused by a variety of new infecting organisms, in contrast to bacterial persistence in which the same infecting organism is always isolated. However, the most common general pathogenic species is E. coli, which occurs in many different serotypes. Therefore, recurrent E. coli UTI does not equate to infection with the same organism.
3.9.2.4. Classification according to symptoms
Children may have typical or atypical symptoms regarding a UTI. In neonates and infants the most commen symptoms are fever, vomiting, lethargy and/or irritability. Infants and children may have non-specific signs such as poor appetite, failure to thrive, lethargy, irritability, vomiting or diarrhoea. Toilet trained children may report cystitis symptoms along with fever/flank pain.
Asymptomatic bacteriuria indicates attenuation of uropathogenic bacteria by the host, or colonisation of the bladder by non-virulent bacteria that are incapable of activating a symptomatic response (no leukocyturia, no symptoms). Asymptomatic UTI includes leukocyturia but no other symptoms.
Symptomatic UTI, includes irritative voiding symptoms, suprapubic pain (cystitis), fever and malaise (pyelonephritis). Cystitis may represent early recognition of an infection destined to become pyelonephritis, or bacterial growth controlled by a balance of virulence and host response.
3.9.2.5. Classification according to complicating factors
In uncomplicated UTI, infection occurs in a patient with a morphologically and functionally normal upper and lower urinary tract, normal renal function and competent immune system. This category includes mostly isolated or recurrent bacterial cystitis and is usually associated with a narrow spectrum of infecting pathogens that are easily eradicated by a short course of oral antimicrobial agents. Patients can be managed on an outpatient basis, with an emphasis on documenting resolution of bacteriuria, followed by elective evaluation for potential anatomical or functional abnormalities of the urinary tract [440].
A complicated UTI occurs in children with known mechanical or functional pathology of the urinary tract. Mechanical obstruction is commonly due to the presence of posterior urethral valves, strictures or stones, independent of their location. Functional obstruction often results from lower urinary tract dysfunction (LUTD) of either neurogenic or non-neurogenic origin and dilating VUR. Patients with complicated UTI require hospitalisation and parenteral antibiotics. Prompt anatomical evaluation of the urinary tract is critical to exclude the presence of significant abnormalities [441]. If mechanical or functional abnormalities are present, adequate drainage of the infected urinary tract is necessary.
3.9.3. Diagnostic evaluation
3.9.3.1. Medical history
Medical history includes the question of a primary (first) or secondary (recurring) infection; possible malformations of the urinary tract (e.g. pre- or post-natal US screening); prior operation; family history; and, whether there is constipation or presence of lower urinary tract symptoms (LUTS).
3.9.3.2. Clinical signs and symptoms
Neonates with severe UTI can present with non-specific symptoms (failure to thrive, jaundice, hyperexcitability) and without fever. In neonates it is important to rule out a co-existing meningitis [442]. Urinary tract infection is the cause of fever in 4.1-7.5% of children who present to a paediatric clinic [443,444]. Septic shock is unusual, even with very high fever. Signs of a UTI may be vague and unspecific in small children, but later on, when they are more than two years old, frequent voiding, dysuria and suprapubic, abdominal or lumbar pain can be detected.
3.9.3.3. Physical examination
Physical examination includes a general examination of the throat, lymph nodes, abdomen (constipation, palpable and painful kidney, or palpable bladder), flank, the back (stigmata of spina bifida or sacral agenesis), genitalia (phimosis, labial adhesion, vulvitis, epididymo-orchitis), measurement of body weight and temperature.
3.9.3.4. Urine sampling, analysis and culture
Urine sampling has to be performed before any antimicrobial agent is administered. The technique for obtaining urine for urinalysis as well as culture affects the rate of contamination, which influences interpretation of the results. Especially in early infancy, it can be challenging and depends on the mode of urine sampling [445].
3.9.3.4.1. Urine sampling
Urine must be collected under defined conditions and investigated as soon as possible to confirm or exclude UTI, especially in children with fever. In neonates, infants and non-toilet-trained children, there are four main methods with varying contamination rates and invasiveness to obtain urine:
(1) Plastic bag attached to the cleaned genitalia: Although this technique is most often used in daily practice, contamination rates are high with around 50-60% [446]. It is helpful when the culture results are negative. Also, if the dipstick is negative for both leukocyte esterase and nitrite, or microscopic analysis is negative for both pyuria and bacteriuria, UTI can be excluded without the need for confirmatory culture [447].
(2) Clean-catch urine (CCU) collection: The infant is placed in the lap of a caregiver or member of the nursing staff, who holds a sterile foil bowl underneath the infant’s genitalia. The infant is offered oral fluids and urine collection is awaited [448]. Suprapubic tapping alternated with paravertebral lumbar massage can stimulate sponateous voiding [446,449]. There seems to be a good correlation between the results of urine culture obtained by this method and suprapubic aspiration (SPA), with a false-positive rate of 5% and false-negative rate of 12% [448,450]; however, the contamination rate is higher for CCU with up to 26% compared to catheterisation 10% and SPA 1% [446,451]. In one prospective cohort study in infants below the age of six months, the success rate was 49% and the contamination rate 16% with some differences in the culture results between those obtained by CCU and those by more invasive methods [452].
(3) Transurethral bladder catheterisation: is the fastest and safest method to obtain a reliable urine sample fo microscopic and bacteriological evaluation to rule out or to document a UTI in non-toilet trained infants and children.
(4) Suprapubic bladder aspiration: This is the most invasive but also the most sensitive method to obtain an uncontaminated urine sample in this age group [453,454]. For suprapubic puncture ultrasound imaging should be performed to asses bladder filling. A two-step procedure where the CCU is screened and a catheter or SPA confirmation of the positive screens is used can lead to a reduction in invasive procedures [446,451]. In older, toilet-trained children who can void on command, after carefully retracting the foreskin and cleaning the glans penis in boys and spreading the labia and cleaning the peri-urethral area in girls, the use of clean catch, especially midstream urine, could be an acceptable technique for obtaining urine. After cleaning the urethral meatus and perineum with gauze and liquid soap twice, the risk of contamination was reduced from 23.9% (41/171) to 7.8% (14/171) in a randomised trial [455].
3.9.3.4.2. Urinalysis
There are three methods that are commonly used for urinalysis:
(1) Dipsticks: These are appealing because they provide rapid results, do not require microscopy, and are ready to use. Leukocyte esterase (as a surrogate marker for pyuria) and nitrite (which is converted from dietary nitrates by most Gram-negative enteric bacteria in the urine) are the most frequent markers, and are usually combined in a dipstick test. The conversion of dietary nitrates to nitrites by bacteria takes approximately four hours in the bladder [450,456]. Using only nitrate sticks to screen febrile children < 2 years of age has a too low sensitivity and relevant UTIs can be missed. However, the specificity is high for children at any age [457,458]. In febrile infants < 90 days old urine dipstick tests using CCU samples can be used for screening for a UTI when nitrites and leukocyte esterase combined are used with a sensitivity of 86% and a specificity of
80% [459].
(2) Microscopy: This is the standard method of assessing pyuria after centrifugation of the urine with a threshold of five white blood cells (WBCs) per high-power field (25 WBC/μL) [460]. In uncentrifuged urine,
> 10 WBC/μL has been demonstrated to be sensitive for UTI [461] and this could perform well in clinical situations [462]. However, this is rarely done in an outpatient setting. No significant differences was found between dipsticks and microscopy testing for UTI [458]. A meta-analysis showed, that only microscopy with Gram staining has a higer sensitivity compared to dipsticks [463].
(3) Flow imaging analysis technology: This is being used increasingly to classify particles in uncentrifuged urine specimens [464]. The numbers of WBCs, squamous epithelial cells and red cells correlate well with those found by manual methods [450]. Flow cytometry-based bacterial and leukocyte count analysis when using a cut-off value of 250 bacteria/uL in the presence of leukocyturia has a sensitivity of 0.97 and specificity of 0.91 for diagnosing UTI [465].
3.9.3.4.3. Urine culture
After negative results for dipstick, microscopic or automated urinalysis, urine culture is generally not necessary, especially if there is an alternative source of fever. If the dipstick result is positive, confirmation by urine culture is strongly recommended.
It is unclear what represents a significant UTI. In patients with a severe UTI, > 105 cfu/mL can be expected. However, the count can vary and be related to the method of specimen collection, diuresis, and time and temperature of storage until cultivation occurs [426]. Clean-catch urine, midstream and catheterisation urine cultures can be considered positive as 103 - 104 cfu/mL in a monoculture, and any counts obtained after SPA should be considered as significant. Mixed cultures are indicative of contamination. In febrile children
< 4 months of age a cut-off value of 103 cfu/mL can be used when clinical and laboratory findings match and a correct sampling method has been used [466].
A negative culture with the presence of pyuria may be due to incomplete antibiotic treatment, urolithiasis, or foreign bodies in the urinary tract, and infections caused by Mycobacterium tuberculosis or Chlamydia trachomatis.
A flowchart was developed as guidance during the basic diagnostic evaluation and subsequent management of febrile children with clinical symptoms of UTI, Figure 4.
Figure 4: Diagnostic evaluation and subsequent management of a febrile child with clinical symptoms of UTI CRP = C-reactive protein; AB = antibiotic.
CRP = C-reactive protein; AB = antibiotic.
3.9.3.5. Imaging
3.9.3.5.1. Ultrasound
Renal and bladder US within 24 hours is advised in infants with febrile UTI to exclude obstruction of the upper and lower urinary tract. Abnormal results are found in 15% of cases, and 1-2% have abnormalities that require prompt action (e.g., additional evaluation, referral or surgery) [447]. When a renal US is performed in all children presenting with a UTI, 7% will have an abnormal US warranting further investigations [467]. The sensitivity to detect high-grade VUR with US was found to be 0.59 (CI: 0.45-0.72) with a specificity of 0.79 (CI: 0.65-0.87) [468]. Renal US should be performed before and after voiding. Post-void residual (PVR) urine should be measured in toilet-trained children to exclude voiding abnormalities as a cause of UTI. Elevated PVR urine volume predicts recurrence of UTIs in toilet-trained children [469]. When peri-renal or psoas abcesses or renal masses are seen on US, it is important to consider xanthogranulomatous pyelonephritis, and subsequent CT imaging is proposed [470].
3.9.3.5.2. Radionuclide scanning/MRI
Changes in dimercaptosuccinic acid (DMSA) clearance during acute UTI indicate pyelonephritis or parenchymal damage, correlated with the presence of dilating reflux and the risk of further pyelonephritis episodes, breakthrough infections [471] and future renal scarring. In the acute phase of a febrile UTI (up to four to six weeks), DMSA-scan can demonstrate pyelonephritis by perfusion defects. Renal scars can be detected after three to six months [472]. Diffusion-weighted MRI has shown to accurately diagnose acute pyelonephritis and reveal late renal scars and could be an alternative to DMSA; therefore, avoiding radion burden [473]. The average effective radiation dose of a single DMSA scan was 2.84 (1-12) mSv in one study [474]. These findings are different in neonates. After the first symptomatic, community-acquired UTI, the majority of renal units with VUR grade III or higher had normal early DMSA scanning [475]. The sensitivity of the DMSA scan to detect VUR is 0.75 (CI: 0.67-0.81) with a specificity of 0.48 (CI: 0.38-0.57), and a negative DMSA scan resulting in a very low probability of high-grade VUR [476].
3.9.3.5.3. Voiding cystourethrography/urosonography
The optimum method to exclude or confirm VUR is VCUG. The timing of VCUG does not influence the presence or severity of VUR [477]. Performance of early VCUG in patients with proven sterile urine does not cause any significant morbidity [478]. Using harmonic voiding urosonography may be an alternative to the standard VCUG avoiding radiation [479]. Visulatisation of the urethra may be difficult with this technique.
It is important to diagnose high-grade VUR after the first UTI since this is an important risk for renal scarring. On the other hand, physicians want to avoid unnecessary VCUG investigations at the same time, given its invasive character and radiation burden [467,480]. Various studies have investigated the risk factors for high-grade VUR and a top down approach is feasible. The most important risk factors for high-grade VUR and subsequent renal scarring are: abnormal renal US, high fever UTI and non-E. Coli infections. Different top down strategies with selective VCUG investigations have been proposed [481-485]. Based on these studies we recommend the following updated diagnostic strategy (see Figure 5).
Figure 5: Diagnosis strategy for first febrile UTI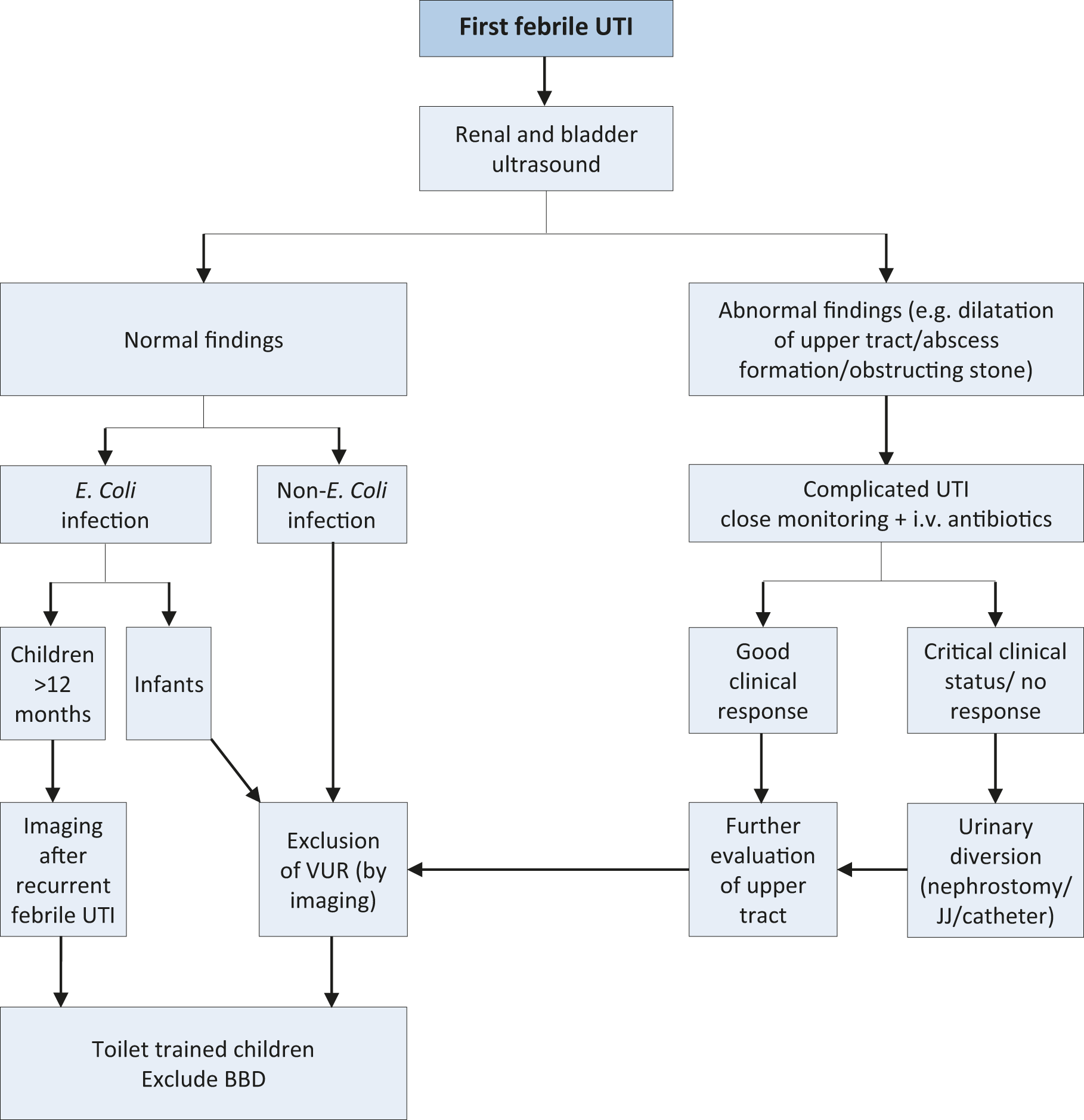 UTI = urinary tract infection; VUR = vesicoureteral reflux; i.v. = intravenous; BBD = bladder and bowl dysfunction.
UTI = urinary tract infection; VUR = vesicoureteral reflux; i.v. = intravenous; BBD = bladder and bowl dysfunction.
3.9.4. Management
3.9.4.1. Administration route of antibacterial therapy
The choice between oral and parenteral therapy should be based on patient age; clinical suspicion of urosepsis; illness severity; refusal of fluids, food and/or oral medication; vomiting; diarrhoea; non-compliance; and complicated pyelonephritis (e.g. urinary obstruction). As a result of the increased incidence of urosepsis and severe pyelonephritis in newborns and infants aged less than two months, parenteral antibiotic therapy is recommended. Electrolyte disorders with life-threatening hyponatraemia and hyperkalaemia based on pseudohypoaldosteronism can occur in these cases [486,487].
The choice of agent is also based on local antimicrobial sensitivity patterns, and should later be adjusted according to sensitivity-testing of the isolated uropathogen [450]. Not all available antibiotics are approved by national health authorities, especially in infancy. When recent urinary cultures are available use these sensitivity patterns in the choice for treatment. In children who require intravenuous treatment tobramycin or gentamicin is recommended if there is normal kidney function. When abnormal kidney function is suspected, ceftriaxon or cefotaxime are alternative treatment options. In children who can receive oral treatment without any known resistant urinary cultures, cefixime or amoxicillin-clavulanate are the empirical treatment options [488]. Some studies have demonstrated that once daily parenteral administration of gentamicin or ceftriaxone in a day treatment centre is safe, effective and cost-effective in children with UTI [489-491]. Delaying treatment in children with a febrile UTI for more than 48-72 hours increases the risk of renal scars [432,476].
3.9.4.2. Duration of therapy
Prompt adequate treatment of UTI can prevent the spread of infection and renal scarring. In newborns and young infants with a febrile UTI, up to 20% may have a positive blood culture [436,441]. Children with bacteremia did not show significant clinical differences with non-bacteremic infants, but did receive longer parental treatment [492]. In late infancy, there are no differences between strategies regarding the incidence of parenchymal scars, as diagnosed with DMSA scan [493]. Outcomes of short courses (one to three days) are inferior to seven to fourteen-day courses [450]. However, a simple cystitis can be treated with three to five days of antibiotics [488]. No significant difference in recurrent UTIs and rehospitalisation was found between seven day parental treatment and longer regimens for bacteremic UTI in younger infants [494]. In young infants a short course of parental treatment with early conversion to oral antibiotics may be considered. The use of exclusively oral therapy with a third-generation cephalosporin (e.g. cefixime or ceftibuten) has been demonstrated to be equivalent to the usual two to four days intravenous therapy followed by oral treatment [495-498]. Similar data have been shown for amoxicillin-clavulanate [499]. If ambulatory therapy is chosen, adequate surveillance, medical supervision and, if necessary, adjustment of therapy must be guaranteed. In the initial phase of therapy, a close ambulant contact to the family is advised [500].
In complicated UTI, uropathogens other than E. coli, such as Proteus mirabilis, Klebsiella spp., Pseudomonas aeruginosa, enterococci and staphylococci are more often the causative pathogens [441]. A temporary urinary diversion (transurethral catheter, suprapubic cystostomy, percutaneous nephrostomy or ureteral stenting) might be required in case of failure of conservative treatment in obstructive uropathy. Children with acute focal bacterial nephritis often present without pyyuria and significant bacteriuria. For the majority of children, the pathogenesis is related to ascending infection due to pre-existing uropathy, especially VUR or urinary obstruction. Initial management consists of broad-spectrum antibiotics with good tissue penetration. A treatment regimen of a total of three weeks with initial intravenous and subsequently oral therapy tailored to the pathogen identified in culture is recommended [501].
3.9.4.3. Antimicrobial agents
There is a great difference in the prevalence of antibiotic resistance of uropathogenic E. coli in different countries, with increased high resistance patterns in countries outside of the Organisation for Economic Co-operation and Development (OECD) [502]. There are upcoming reports of UTIs caused by extended spectrum ß-lactamase-producing enterobacteriaceae (ESBL) in children, with pooled numbers of UTI caused by ESBL producing bacteria of around 14% [503]. Within OECD countries the prevalence of resistance was 53% for ampicillin, 24% for trimethoprim, 8% for co-amoxiclav, 2% for ciproxin and 1% for nitrofurantoin [502]. Several risk factors and determinants for UTIs caused by ESBL and non-E. coli bacteriae have been identified: history of infection, recent hospitalisation, short-term exposure to antiobiotics, and prophylaxis [502,504,505].Overall, oral nitrofurantoin seems to be a good empirical choice in the treatment of cystitis [506].
The choice of antibiotics should be guided by good antibiotic stewardship. It is important to be aware of the local resistance patterns. These are variable between countries and moreover between hospitals. Local antibiotic protocols and web-based recommendations can guide the choice for type of antibiotic therapy. The individual patient’s previous urine cultures should also be taken into account in this decision. The daily dosage of antibiotics is depended on the age, weight of the child as well as on renal and liver function.
3.9.4.4. Preventative measures
Recurrent UTIs are problematic because the symptoms are bothersome to children and recurrent febrile UTIs will also result in renal scarring [434]. Therefore, it is important to prevent the incidence of recurrent UTIs.
3.9.4.4.1. Chemoprophylaxis
Chemoprophylaxis is commonly used to prevent UTIs in children. However, with the increasing bacterial resistance numbers, it should be carefully considered which patients should receive antibacterial prophylaxis. The evidence for the use of antibacterial prophylaxis has been conflicting. Its use causes a reduction of the number of recurrent symptomatic UTIs, but long-term use of antibacterial prophylaxis has also been associated with increased microbial resistance [438,507]. Its use did not reduce newly acquired renal damage in children with first or second UTI [507]. However, when used in patients with anatomic abnormalities of the urinary system a reduction in UTIs and subsequent renal scarring was shown [438,507]. In children with BBD and VUR, a benefit was seen in the reduction of recurrent UTI with the use of antomicrobial prophylaxis [508,509] (see also Chapter 3.14 on VUR). For the specific group of patients with incomplete bladder emptying with properly performed clean intermittent catheterisation but still suffering from recurrent UTIs the intravesical application of gentamycin has proven to be effective [510].
Table 1:Drugs for antibacterial prophylaxis*
Substance | Prophylactic dosage (mg/kg bw/d) | Limitations in neonates and infants |
Trimethoprim** | 2 | Not recommended under 6 weeks of age |
Trimethoprim | 1-2 10-15 | Not recommended under two months of age |
Sulfamethoxazole | 1-2 | Until three months of age |
Nitrofurantoin** | 1-2 | Not recommended under two months of age |
Cefaclor | 10 | No age limitations |
Cefixim | 2 | Preterms and newborns |
* Reproduced with permission from the International Consultation on Urological Diseases (ICUD), InternationalConsultation on Urogenital Infections, 2009. Copyright© by the European Association of Urology .511
** Substances of first choice are nitrofurantoin and trimethoprim. In exceptional cases, oral cephalosporin can be used.
3.9.4.4.2. Dietary supplements
Cranberry, mostly as juice, has been shown to prevent UTIs in healthy children, while in children with urogenital abnormalities, cranberries appear to be just as effective as antibiotic prophylaxis [512]. The results for probiotics are somewhat more conflicting, with one systematic review not ruling out any effect [304] and a RCT showing promising results in children with normal urogenital anatomy [513]. A meta-analysis could not demonstrate a beneficial effect, only as an adjuvant to antibiotic prophylaxis [514].
Other supplements of interest were Vitamin A, which showed promising results in preventing renal scarring in children with acute pyelonephritis [515,516]. The use of Vitamin E could possibly improve the symptoms of UTI [517]. More studies into these supplements are warranted.
3.9.4.4.3. Preputium
A risk reduction of recurrent UTI regarding the preputium has been shown in two studies. When a physiologic phimosis is present in boys with a UTI the use of steroid cream significantly reduced recurrent UTIs [43]. In boys with recurrent UTIs and hydronephrosis present, ten boys would need to be circumcised to prevent one UTI [41].
3.9.4.4.4. Bladder and bowel dysfunction
Bladder and bowel dysfunction is a risk factor for which each child with UTI should be screened upon presentation [429]. Normalisation of micturition disorders or bladder overactivity is important to lower the rate of UTI recurrence. If there are signs of BBD at infection-free intervals, further diagnosis and effective treatment are strongly recommended [509]. Treatment of constipation leads to a decrease in UTI recurrence and a multidisciplinary approach is recommended [429,508,509]. Therefore, exclusion of BBD is strongly recommended in any toilet-trained child with febrile and/or recurrent UTI, and it should be treated (For treatment see chapter 3.10 on LUTS).
3.9.4.5. Monitoring of UTI
With successful treatment, urine usually becomes sterile after 24 hours, and leukocyturia normally disappears within three to four days. Normalisation of body temperature can be expected within 24-48 hours after the start of therapy in 90% of cases. In patients with prolonged fever and failing recovery, treatment-resistant uropathogens or the presence of congenital uropathy or acute urinary obstruction should be considered. Repeated US examination is recommended in these cases.
Procalcitonin (among other laboratory inflammatory parameters such as C-reactive protein and leukocyte count) can be used as reliable serum marker for early prediction of renal parenchymal inflammation [518]. A cut-off value of serum procalcitonin of 1.0 ng/mL has been shown to be predictive of acute pyelonephritis in young children [519]. In patients with febrile UTI, serum electrolytes and blood cell counts should be followed up.
3.9.5. Summary of evidence and recommendations for the management of UTI in children
Summary of evidence | LE |
Urinary tract infection represents the most common bacterial infection in children less than 2 years of age. The incidence varies depending on age and sex. | 1b |
Classifications are made according to the site, episode, severity, symptoms and complicating factors. For acute treatment, site and severity are most important. | 2b |
The number of colony forming units (cfu) in the urine culture can vary, however, any colony count of one specimen indicates a high suspicion for UTI. | 2b |
Due to increasing resistance numbers good antibiotic stewardship should guide the choice of antibiotics, taking into account local resistance patterns, old urine cultures (when available) and clinical parameters. | 2a |
Preventive measures against recurrent UTIs include: chemoprophylaxis (oral and intravesical), cranberries, probiotics and Vitamin A and E. | 2a |
Urinalysis by dipstick yields rapid results, but it should be used with caution. Microscopic investigation is the standard method of assessing pyuria after centrifugation. | 2a |
During acute UTI both DMSA and diffusion-weighted MRI can confirm pyelonephritis or parenchymal damage. | 2a |
Recommendations | LE | Strength rating |
Take a medical history, assess clinical signs and symptoms and perform a physical examination to diagnose children suspected of having a urinary tract infection (UTI). | 3 | Strong |
Exclude bladder and bowel dysfunction in any toilet-trained child with febrile and/or recurrent UTI. | 3 | Strong |
Clean catch urine can be used for screening for UTI. Bladder catheterisation and suprapubic bladder aspiration to collect urine can be used for urine cultures. | 2a | Strong |
Do not use plastic bags for urine sampling in non-toilet-trained children since it has a high risk of false-positive results. | 2a | Strong |
Midstream urine is an acceptable technique for toilet-trained children. | 2a | Strong |
The choice between oral and parenteral therapy should be based on patient age; clinical suspicion of urosepsis; illness severity; refusal of fluids, food and/or oral medication; vomiting; diarrhoea; non-compliance; and complicated pyelonephritis. | 2a | Strong |
Treat febrile UTIs with four to seven day courses of oral or parenteral therapy. | 1b | Strong |
Treat complicated febrile UTI with broad-spectrum antibiotics. | 1b | Strong |
Offer long-term antibacterial prophylaxis in case of high susceptibility to UTI and risk of acquired renal damage and lower urinary tract symptoms. | 1b | Strong |
In selected cases consider dietery supplements as an alternative or add-on preventive measure. | 2a | Strong |
In infants with febrile UTI use renal and bladder ultrasound to exclude obstruction of the upper and lower urinary tract within 24 hours. | 2a | Strong |
In infants, exclude vesicoureteric reflux (VUR) after first epidose of febrile UTI with a non-E. Coli infection. In children more than one year of age with an E. Coli infection, exclude VUR after the second febrile UTI. | 2a | Strong |
3.10. Day-time lower urinary tract conditions
3.10.1. Terminology, classification, epidemiology and pathophysiology
Urinary incontinence in children may be caused by congenital anatomical or neurologic abnormalities such as ectopic ureter, bladder exstrophy or myelomeningocele (MMC). In many children, however, there is no such obvious cause for the incontinence, and they are referred as having functional bladder problems. The most recent International Children’s Continence Society (ICCS) document suggests using the term day-time lower urinary tract (LUT) conditions to group together all functional bladder problems in children.
Normal storage and emptying of the bladder at a socially accepted place and time is mostly achieved by age three to four. Children with LUT conditions would present with failure to achieve continence (being still wet after the age of four), urgency, weak stream, hesitancy, frequency and accompanied UTIs. Isolated night-time wetting without any day-time symptoms is known as ‘enuresis’ and considered as a different entity (see chapter 3.11) [520].
As different studies have used varying definitions and criteria, it is difficult to give reliable percentages regarding the incidence of this problem. Reported prevalence ranges widely from 1% to 20% [521-529]. Due to increasing awareness and better access to specialised health care, the prevalence seems to be increasing [530,531].
Lower urinary tract conditions in children may be due to disturbances of the filling phase, the voiding phase or a combination of both in varying severity. Mainly the conditions are divided into either overactive bladder (OAB) or dysfunctional voiding. They can, of course, coincide and one may even be causative of the other. Dysfunctional bowel emptying may also be part of the clinical problems and BBD is the term used to cover concomitant bladder and bowel disturbances.
Lower urinary tract conditions are considered to be the result of incomplete or delayed maturation of the bladder sphincter complex. The pons is considered to be responsible for detrusor sphincter co-ordination while the cortical area is responsible for inhibition of the micturition reflex and voluntary initiation of micturition. Therefore overactivity would be the result of delayed maturation of cortical control, while dysfunctional voiding would be the result of non-maturation of the co-ordination. Detrusor overactivity should not be considered as a sole bladder based problem but more a symptom of a centrally located dysfunction affecting bladder, bowel and even mood and behaviour [532].
A link between LUT and behavioural disorders such as ADHD (attention deficit/ hyperactivity disorder) has also been shown [533-535].
3.10.1.1. Filling-phase (storage) dysfunctions
In filling-phase dysfunctions, the detrusor can be overactive, as in OAB, or underactive, as in underactive bladder (UAB). Overactivity of the bladder is the most common problem, seen mostly around five to seven years of age. This may lead to disturbances characterised by urgency, frequency and at times urgency incontinence. Some children habitually postpone micturition leading to voiding postponement. Therefore, holding manoeuvres such as leg crossing and squatting can often be seen in this group. Recurrent UTIs are common and high-pressure state of the bladder can be a cause of VUR. Constipation can be an additional aetiological factor, which needs to be assessed. In children with an underactive detrusor, voiding occurs with reduced or minimal detrusor contractions with post-void residuals. Urinary tract infections, straining to void, constipation and incontinence is common. Incontinence often occurs when the bladder is over-distended in the form of overflow incontinence.
3.10.1.2. Voiding-phase (emptying) dysfunctions
In voiding-phase (emptying), incomplete relaxation or tightening of the sphincteric mechanism and pelvic floor muscles results in staccato voiding pattern (continuous urine flow with periodic reductions in flow rate precipitated by bursts of pelvic floor activity) or an interrupted voiding pattern (unsustained detrusor contractions resulting in infrequent and incomplete voiding, with micturition in fractions). The general term for this condition is dysfunctional voiding and is associated with elevated bladder pressure and PVR. Symptoms will vary depending on the severity of inco-ordination between bladder and the sphincter. Staccato voiding is in less severe forms and interrupted voiding and straining is in more severe forms. The co-existence of constipation and LUTD and recurrent UTI is well described [536]. There is no evidence to conclude if bladder problems or bowel problems are the leading cause. The prevalence of constipation in older children varies from 5 to 27%. Approximately 90% of them being functional constipation without an organic cause In children with functinal constipation the prevelance of bladder symptoms have been shown to be as high as 64% [537,538].
In incomplete emptying, high voiding pressures generated by bladder working against a functional obstruction caused by non-relaxing sphincter may induce not only UTIs but also VUR. It is been shown that LUTD is more significant for the occurrence of UTI than VUR itself [539]. In the majority of children with dysfunctional voiding the recurrent infections disappear following successful treatment, which confirms the hypothesis that dysfunctional voiding is the main factor responsible for the infections. Spontaneous resolution of VUR may also be seen after successful treatment of dysfunctional voiding.
3.10.2. Diagnostic evaluation
The evaluation of LUT conditions includes medical and voiding history (bladder diaries and structured questionnaires), a physical examination, a urinalysis, and uroflowmetry with PVR. The upper urinary tract (UUT) needs to be evaluated in children with recurrent infections and dysfunctional voiding. Uroflowmetry can be combined with pelvic floor electromyography to demonstrate overactivity of the pelvic floor muscles during voiding. Urodynamic studies are usually reserved for patients with therapy resistant dysfunctional voiding and those not responding to treatment who are being considered for invasive treatment [535,540-543].
In addition to a comprehensive medical history a detailed voiding diary provides documentation of voiding and defecation habits, frequency of micturition, voided volumes, night-time urine output, number and timing of incontinence episodes, and fluid intake. A voiding diary should at least be done for two days, although longer observation periods are preferred. A voiding diary provides information about storage function and incontinence frequency, while a pad test can help to quantify the urine loss. In the paediatric age group, where the history is taken from both the caregivers and child together, a structured approach is recommended using a questionnaire. Many signs and symptoms related to voiding and wetting will be unknown to the caregivers and should be specifically requested, using the questionnaire as a checklist. Some symptom scorings have been developed and validated [544,545]. Although the reliability of questionnaires are limited they are practical in a clinical setting to check the presence of symptoms and have also been shown to be reliable to monitor the response to treatment. History taking should also include assessment of bowel function. For evaluation of bowel function in children, the Bristol Stool Scale is an easy-to-use tool [546,547].
Urinalysis and urinary culture are essential to evaluate for UTI. Since transient voiding symptoms are common in the presence of UTI, exclusion of UTI is essential before further management of symptoms. During clinical examination, genital inspection and observation of the lumbosacral spine and the lower extremities are necessary to exclude obvious uropathy and neuropathy.
Uroflowmetry with PVR evaluates the emptying ability, while an UUT US screens for (secondary) anatomical changes. A flow rate which reaches its maximum quickly and levels off (‘tower shape’) may be indicative of over-active bladder whereas interrupted or staccato voiding patterns may be seen in dysfunctional voiding. Plateau uroflowmetry patterns are usually seen in anatomic obstruction of flow. A single uroflowmetry test may not always be representative of the clinical situation and multiple uroflowmetry tests, which all give a similar result, are more reliable. Uroflowmetry examination should be done when there is desire to empty the bladder and the voided volume should at least be 50% of the age-expected capacity [(age in years) + 1] x 30 mL for the children. While testing the child in a clinical environment, the impact of stress and mood changes on bladder function should also be taken into account [548,549].
In the case of treatment failure re-evaluation is warranted and (video)-urodynamic (VUD) studies and neurological evaluation may be considered. Sometimes, there are minor, underlying, urological or neurological problems, which can only be suspected using VUD. In these cases, structured psychological interviews to assess social stress should be added [550] (LE: 1b).
Video-urodynamics may also be used as initial investigational tool in patients with suspicion of reflux. In this case reflux may be observed along with bladder dynamics. In the case of anatomical problems, such as posterior urethral valve (PUV) problems, syringocoeles, congenital obstructive posterior urethral membrane (COPUM) or Moormann’s ring, it may be necessary to perform cystoscopy with treatment. If neuropathic disease is suspected, MRI of the lumbosacral spine and medulla can help to exclude tethered cord, lipoma or other rare conditions.
3.10.3. Management
The treatment of LUTD involves a multimodal approach, involving strategies such as behavioural modification, and anticholinergic medication along with underlying and potentially complicating conditions such as constipation and UTIs.
Behavioural modification, mostly referred to as urotherapy, is a term which covers all non-pharmacological and non-surgical treatment modalities. It includes standardisation of fluid intake, bowel management; timed voiding and basic relaxed voiding education. The child and family are educated about normal bladder function and responses to urgency. Voiding regimens are instituted and UTIs and any constipation are treated. Treatment is aimed at optimising bladder emptying and inducing full relaxation of the urinary sphincter or pelvic floor prior to and during voiding.
Strategies to achieve these goals include:
1. Information and demystification, which includes explanation about normal LUT function and how a particular child deviates from normal function.
2. Instructions about what to do about the problem:
Regular voiding habits, sound voiding posture, pelvic floor awareness and training to relax pelvic floor and avoiding holding manoeuvres.
Lifestyle advice, regarding fluid intake, prevention of constipation, etc.
Registration of symptoms and voiding habits using bladder diaries or frequency-volume charts.
Support and encouragement via regular follow-up by the caregiver.
Recurrent UTIs and constipation should also be treated and prevented during the treatment period. In case of combined BBD it is advised to treat the bowel dysfunction first [530] as LUTS may disappear after successful management of bowel dysfunction.
Addition of other strategies, as below, may be needed:
- Pelvic floor muscle awareness practices with repeated sessions of biofeedback visualisation of uroflow curves and/or pelvic floor activity and relaxation.
- Clean intermittent self-catheterisation for large PVR volumes of urine.
- Antimuscarinic drug therapy if detrusor overactivity is present.
- If the bladder neck is associated with increased resistance to voiding, α-blocker drugs may be introduced.
Treatment efficacy can be evaluated by improvement in bladder emptying and resolution of associated symptoms. Controlled studies of the various interventions are needed. As with detrusor overactivity, the natural history of untreated dysfunctional voiding is not well delineated and optimum duration of therapy is poorly described. A high success rate has been described for urotherapy programmes, independent of the components of the programme. However, the evidence level is low as most studies of urotherapy programmes are retrospective and non-controlled [551]. A recent Cochrane analysis found very little evidence that can help to make evidene-based treatment decisions [552].
3.10.3.1. Specific interventions
As well as urotherapy, there are some specific interventions, including physiotherapy (e.g. pelvic floor exercises), biofeedback, alarm therapy and neuromodulation. Although good results with these treatment modalities have been reported, the level of evidence remains low, since only a few RCTs were published [553-559].
A systematic review reports that biofeedback is an effective, non-invasive method of treating dysfunctional voiding, and approximately 80% of children benefited from this treatment. However, most reports were of low level of evidence and studies of more solid design such as RCTs should be conducted [560]. A more recently published multicentre controlled trial of cognitive treatment, placebo, oxybutynin and bladder and pelvic floor training did not report better results with oxybutynin and pelvic floor training compared to standard urotherapy [550] (LE: 1b).
Two RCTs on underactive bladder without neurophatic disease have recently been published. Transcutaneous interferential electrical stimulation and animated biofeedback with pelvic floor exercise have been shown to be effective [561,562]. In some cases, pharmacotherapy may be added. Some studies on orthosympathicomimetics have been published with a low level of evidence [563].
Overactive bladder is common in the paediatric population. Although a stepwise approach starting with behavioural therapy is advised, antimuscarinic agents remain the mainstay of medical treatment for OAB. Oxybutynin is the most commonly used antimuscarinic in the paediatric population. The response to antimuscarinics varies and many children experience serious side effects. Although there have been reports about the use of tolterodine, fesoterodine, trospium, propiverine, and solifenacin in children, to date, most of them are off-label depending on age and national regulations. A few RCTs have been published, one on tolterodine showed safety but not efficacy [564], while another on propiverine showed both safety and efficacy [565] (LE:1). The recent study on solifenacin showed its efficacy with side effects like constipation and electrocardiogram changes [566].
The difference in results is probably due to study design. Despite the low level of evidence for the use of anticholinergics and antimuscarinics, their use is recommended because of the large number of studies reporting a positive effect on OAB symptoms. Although α-blocking agents are used occasionally, an RCT showed no benefit [567]. Botulinum toxin injection seems promising, but can only be used off-label [568].
A meta-analysis reports that neuromodulation therapy may lead to better partial improvement of nonneurogenic OAB; however, it may not render a definitive complete response. Office-based neuromodulation seems more efficacious than self-administered neuromodulation [569]. These new treatment modalities can only be recommended for standard therapy-resistant cases [570]. Despite early successful treatment, there is evidence that there is a high recurrence rate of symptoms in the long term which necessitates long-term follow-up [571]. In addition, many patients may present later in adulthood with different forms of LUTD [572].
3.10.4. Summary of evidence and recommendations for the management of day-time lower urinary tract conditions
Summary of evidence | LE |
The term ‘bladder bowel dysfunction’ should be used rather than ‘dysfunctional elimination syndrome and voiding dysfunction’. | 4 |
Day-time LUTS has a high prevalence (1% to 20%). | 2 |
Recommendations | LE | Strength rating |
Use two day voiding diaries and/or structured questionnaires for objective evaluation of symptoms, voiding drinking habits and response to treatment. | 2 | Strong |
Use a stepwise approach, starting with the least invasive treatment in managing day-time lower urinary tract dysfunction in children. | 4 | Weak |
Initially offer urotherapy involving bladder rehabilitation and bowel management. | 2 | Weak |
If bladder bowel dysfunction is present, treat bowel dysfunction first, before treating the lower urinary tract condition. | 2 | Weak |
Use pharmacotherapy (mainly antispasmodics and anticholinergics) as second-line therapy in overactive bladder. | 1 | Strong |
Use antibiotic prophylaxis if there are recurrent infections. | 2 | Weak |
Re-evaluate in case of treatment failure; this may consist of (video) urodynamics magnetic resonance imaging of lumbosacral spine and other diagnostic modalities, guiding to off-label treatment which should only be offered in highly experienced centres. | 3 | Weak |
3.11. Monosymptomatic nocturnal enuresis – bedwetting
3.11.1. Epidemiology, aetiology and pathophysiology
Monosymptomatic nocturnal enuresis (NE), also known as bedwetting, is defined as an intermittent nocturnal incontinence. It is a relatively frequent symptom in children, 5-10% at seven years of age and 1–2% in adolescents. There is a gender difference in the incidence: two boys to one girl at any age [573]. With a spontaneous yearly resolution rate of 15% (at any age), it is considered as a relatively benign condition [548,574]. Seven out of 100 seven-year-old bedwetting children will continue to wet their bed into adulthood. Nocturnal enuresis is considered primary when a child has not yet had a prolonged period of being dry (six months). The term “secondary NE” is used when a child or adult begins wetting again after having stayed dry.
Non-monosymptomatic NE is defined as the condition of NE in association with day-time lower urinary tracts symptoms (LUTS, recurrent UTIs and/or bowel dysfunction) [574,575]. The presence of constipation has a negative association with bladder capacity [576].
Nocturnal enuresis has significant secondary stressful, emotional and social consequences for the child and their caregivers. A lower quality of life has been reported for children with NE compared to controls and NE can influence relationships with friends and family [577-580]. Therefore, treatment is advised from the age of six to seven years onwards considering mental status, family expectations, social issues, and cultural background.
There is a clear hereditary factor in NE. If none of the parents or their immediate relatives has suffered from bedwetting, the child has a 15% chance of wetting its bed. If one of the parents, or their immediate relatives have suffered from bedwetting, the chance of bedwetting increases to 44%, and if both parents have a positive history the chance increases to 77%. However, from a genetic point of view, enuresis is a complex and heterogeneous disorder. Loci have been described on chromosomes 12, 13 and 22 [575].
Children with NE are considered deep but poor sleepers due to high arousal thresholds and frequently disturbed sleep. High arousal threshold is the most important pathophysiological factor in the aetiology of NE: the child does not wake up when the bladder is full. Full night polysomnographic recordings support this hypothesis by demonstrating the disruption of children’s sleep microstructure [581]. In addition to the high arousal threshold, there needs to be an imbalance between night-time urine output and night-time bladder capacity [548,574,575]. Recently, attention has been given to the chronobiology of micturition in which the existence of a circadian clock in kidney, brain and bladder is postulated [582].
A high incidence of comorbidity and correlation between nocturnal urine production and sleep disordered breathing, such as obstructive sleep apnoea, has been found and investigated [583]. Symptoms such as habitual snoring, apnoeas, excessive sweating at night and mouth breathing in the patient history or via sleep questionnaires, such as the BEARS questionnaire [584], can lead to the detection of sleep disorders and/or adenotonsillar hypertrophy. When present, a consultation with the ENT specialist can be considered [585].
Obesity is associated with a higher incidence of NE and a lower efficacy for treatment [586]. The presence of allergic diseases has been recognised as a risk factor of NE and with a greater risk for more allergic episodes [587-589].
It is important to consider the child’s and family’s psychological status as primary NE has been associated with psychopathology, such as Attention Deficit Hyperactivity Disorder (ADHD) and depressive symptoms [590,591]. In children with ADHD symptoms of NE are more severe and it is important to inform the child and the parents about a delayed success rate and higher relapse rate compared to children without ADHD [592].
3.11.2. Diagnostic evaluation
The diagnosis is mainly obtained by history-taking. Focused questions to differentiate monosymptomatic vs. non-monosymptomatic, primary vs. secondary, comorbid factors such as behavioural or psychological problems and sleep disorder breathing, should be asked. In addition, a two-day complete micturition and drinking diary, which records day-time bladder function and drinking habits will further exclude comorbid factors such as LUTS and polydipsia.
Specific attention should be made regarding bowel movements as irregular bowel movements can change the diagnosis from monosymptomatic NE to non-monosymptomatic NE. If constipation or faecal incontinence is found (it is reported in up to 20% of children with NE), it should be treated simultaneously, and the family should be informed that constipation can negatively influence treatment outcomes [593,594].
The night-time urine production should be registered by weighing the night-time diapers in the morning and adding the first morning voided volume [595]. The night-time urine production should be recorded over (at least) a two-week period to diagnose an eventual differentiation between a high night-time production (more than 130% of the age expected bladder capacity) vs. a night-time OAB.
A physical examination should be performed with special attention to the back of the child (to exclude any neurological problem), the external genitalia and surrounding skin, as well as to the condition of the clothes (wet underwear or encopresis).
Urine analysis is indicated if there is a sudden onset of bedwetting, a suspicion or history of UTIs, or inexplicable polydipsia.
A uroflowmetry and US is indicated only if there is a history of previous urethral or bladder surgery and presence of daytime urinary symptoms. For further evaluation, see Section 3.10 on Day-time LUT conditions.
There is no clinical indication nor use for a functional MRI (fMRI) in the diagnostic of NE. Research is ongoing, however one of the main issues is the fact that the MRI is performed in an awake state, whereas the NE is a solely event during sleep. The use of fMRI in the elucidation of the NE’s neuropathological mechanisms has not yet been fruitful [596,597].
3.11.3. Management
Before introducing any form of possible treatment, it is of utmost importance to explain the bedwetting condition to the child and the caregivers in order to demystify the problem. Parents should be encouraged to seek medical attention for their bedwetting children and be informed that it is known that the quality of life of parents with a child with NE is negatively impaired. Medical providers assisting families with a child must be aware of this fact and therefore guide parents, by explaining that the key role for treating a child with NE is the ability to understand and the co-operation of the child itself [598].
Since the COVID-19 pandemic promotion of virtual contacts between doctors and patients, it has been shown that telemedicine is a good method for closely monitor patients and can be used for follow-up after treatment [599].
3.11.3.1. Supportive treatment measures
Initially, supportive measures including normal and regular eating and drinking habits should be reviewed, stressing normal fluid intake during the day and reducing fluid intake in the hours before sleep. Keeping a chart depicting wet and dry nights, also called as basic bladder advice, has not been shown to be successful in the early treatment of NE [600]. To assure good sleep quality, specifically in children with NE, it is also recommended to limit the use of electronic devices before bedtime [601].
Referral for psychological support should be advised and followed-up for patients with NE and their families, especially if the NE comorbid factor is developmental, attention or learning difficulties, family problems, parental distress and possible punishment of the child are observed. Parental stress levels are higher compared to parents of non-NE children [602] and anger is found to be the most common parental reaction towards NE children [603], this would explain why childhood traumas such as neglect and abuse are more often seen in children with NE [604]. Psychological interventions with parents of NE children were shown to significantly improve their coping mechanisms [605].
3.11.3.2. Wetting alarm treatment
The nocturnal alarm treatment relies on the use of a device that is activated by getting wet. The goal of this therapeutic approach is that the child wakes up by the alarm, which can be acoustic or tactile, either by itself or with the help of a caregiver. Their method of action is to repeat the awakening and therefore change the high arousal to a low arousal threshold, specifically when a status of full bladder is reached. In the most recent Cochrane review (even though the quality of the included studies was low), several studies have shown that alarm treatment will reduce the number of wet nights a week. An alarm treatment has a higher complete response rate and a low relapse rate compared to no treatment at all [606]. In the event of relapse after initial success, one should actively investigate for OAB [607]. The recommended length of therapy with the alarm treatment continues to be uncertain, varying from 8-12 weeks (ICCS) to 16-20 weeks [608].
Regular follow-up will improve the success. It is of utmost importance that the child plays an active role in the alarm treatment, is willing to continue and understand the purpose of the treatment modality.
3.11.3.3. Medical treatment
If the child and the family would like to act on the high night-time urine production and eventual night-time OAB, they should be able and willing to adjust their drinking habits and take either desmopressin or a combination of desmopressin and an anticholinergic drug.
Success rates of 70% can be obtained with Desmopressin , either as tablets (200-400 μg), or as sublingual Desmopressin oral lyophilisate (120-240 μg). A rare side-effect is water intoxication which can be prevented by adequate water intake. The dosage of 120 ug has been shown to be effective and safe [609]. A structured titration increase up to 240 ug has been shown to be effective [610]. Predictive factors for success with Desmopressin have been identified: older children, in children with fewer wet nights and high night-time urine production [611]. Children that show a good response on low-dose Desmopressin are more likely to show a complete response during the maintenance period [612]. When poor responses are seen on Desmopressin be aware of low compliance [613]. Relapse rates can be high after Desmopressin discontinuation [548], it is unclear if structured withdrawal will result in lower relapse rates [614,615]. A nasal spray is no longer recommended due to the increased risk of overdose [616].
In the event of Desmopressin-resistant treatment for NE or if a suspicion exists for night-time OAB, combination of Desmopressin with anticholinergics is safe and efficient, even after cessation of treatment [617-620]. With night-time OAB a treatment failure to Desmopressin can be explained because of the bladder reservoir dysfunction [621]. There is no indication for monotherapy with an anticholinergic drug [622].
Alarm and Desmopressin treatment have comparable efficacy in achieving >50% reduction in wet nights. Alarms offer superior treatment response (OR: 2.89, 95% CI 1.38 to 6.04) and lower relapse rates (OR: 0.25, 95% CI 0.12 to 0.50) in children [623]. Multimodal treatment can achieve a partial or full response in 80% of children. However, side effects are seen in up to 30% of children [624].
3.11.3.4. Electrical neuromodulation
Several systematic reviews and randomized trials have documented potential benefits of electrical neural stimulation for NE. However, the quality of the included studies was low and different types of electrical neural stimulation, such as intra-anal stimulation and interferential current stimulation have been included [625-628]. The one RCT that compares transcutaneous electrical nerve stimulation to placebo demonstrates no anti-enuretic effect [629].
3.11.3.5. Complementary treatments:
A Cochrane review showed no benefit for treatments such as hypnosis, psychotherapy, acupuncture, chiropractic and medicinal herbs for the treatment of NE [630].
3.11.3.6. Conservative “wait and see” approach
If the child and its family is unable to comply with a treatment, if the treatment options are not possible for the family situation, and if there is no social pressure, a “wait and see” approach can be chosen. However, in this approach, it is important to emphasise the fact that the child should wear diapers at night to ensure a normal quality of sleep [631]. The success rate of wait and see is 15% per year, independent of age. Figure 6 presents stepwise assessment and management options for NE.
Figure 6:A stepwise assessment and management options for NE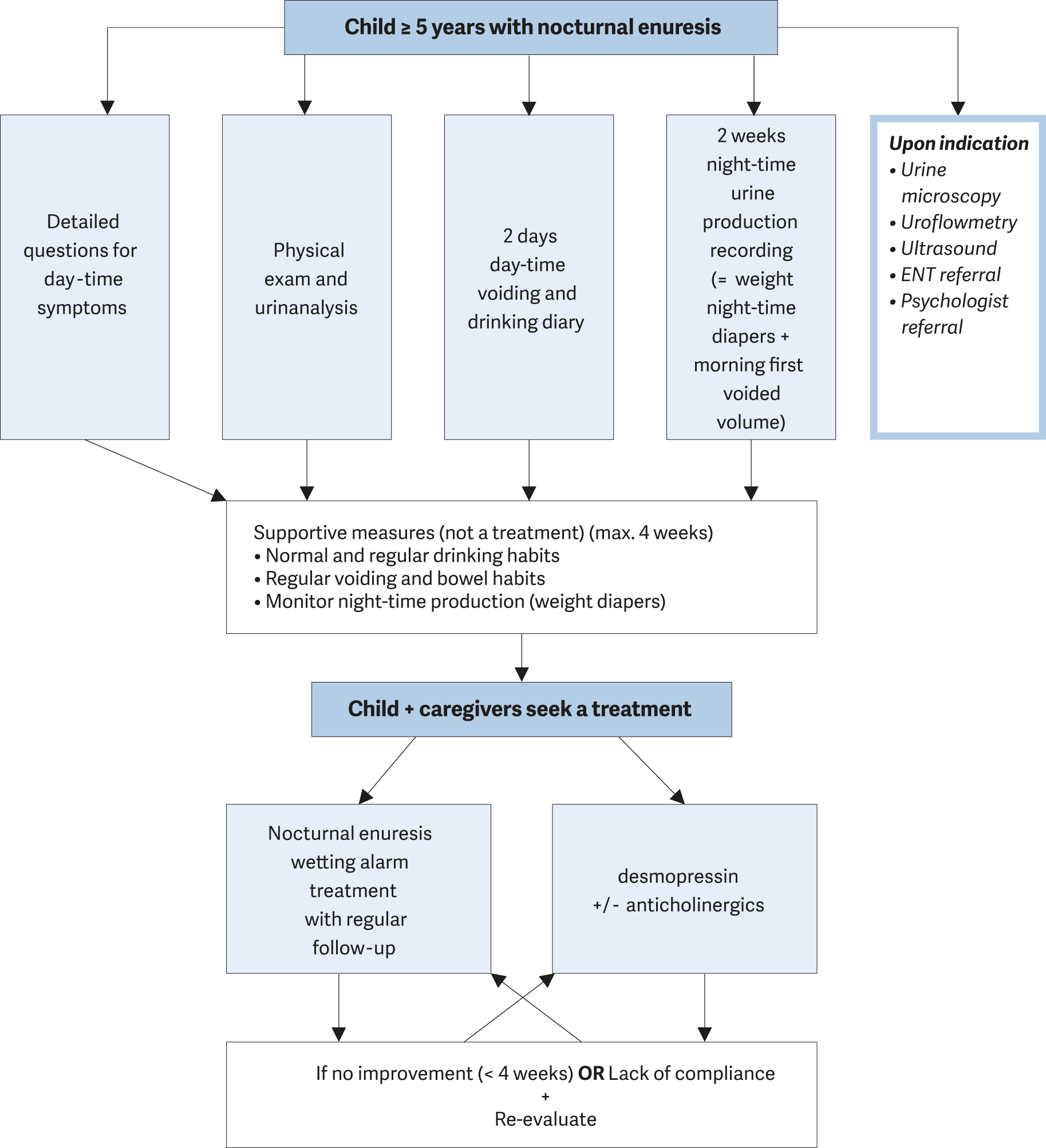 ENT = ear, nose and throat.
ENT = ear, nose and throat.
3.11.4. Summary of evidence and recommendations for the management of monosymptomatic enuresis
Summary of evidence | LE |
Chronobiology of micturition, in which the existence of a circadian clock has been proven in kidney, brain and bladder, and disturbances in this chronobiology play a major role in the pathophysiology of enuresis. | 1 |
Recommendations | LE | Strength rating |
Do not treat children less than five years of age in whom spontaneous cure is likely, but inform the family about the involuntary nature, the high incidence of spontaneous resolution and the fact that punishment will not help to improve the condition. | 2 | Strong |
Use micturition diaries or questionnaires to exclude day-time symptoms. | 2 | Strong |
Perform a urine test to exclude the presence of infection or potential causes such as diabetes insipidus. | 2 | Strong |
Offer supportive measures in conjunction with other treatment modalities, of which pharmacological and alarm treatment are the two most important. | 1 | Strong |
Offer desmopressin in proven night-time polyuria. | 1 | Strong |
Offer alarm treatment in motivated and compliant families. | 1 | Strong |
3.12. Management of neurogenic bladder
3.12.1. Epidemiology, aetiology and pathophysiology
Neurogenic detrusor-sphincter dysfunction (NDSD) can develop as a result of a lesion at any level in the nervous system. This condition contributes to various forms of LUTD, which may lead to incontinence, UTIs, VUR, and ultimately to renal scarring and renal failure requiring dialysis and/or transplantation. Conservative treatment starting in the first year of life is the first choice, however, surgery may be required at a later stage to establish adequate bladder storage, continence and drainage later on [632]. The main goals of treatment concerning the urinary tract are prevention of UTI’s, urinary tract deterioration, achievement of continence at an appropriate age and promoting as good as possible QoL [4,5]. With regard to the associated bowel dysfunction, stool continence, with evacuation at a social acceptable moment, is another goal as well as education and treatment of disturbance in sexual function. Due to the increased risk of development of latex allergy, latex-free products (e.g., gloves, catheters etc.) should be used from the very beginning whenever possible [633].
Neurogenic bladder in children with myelodysplasia presents with various patterns of Detrusor-Sphincter-Dyssynergia with a wide range of severity [634]. About 12% of neonates with myelodysplasia have no signs of neuro-urological dysfunction at birth [635]. Newborns with myelodysplasia who initially have normal urodynamic studies are at risk for neurological deterioration secondary to spinal cord tethering, especially during the first six years of life. Close follow-up of these children is important for the early diagnosis and timely surgical correction of tethered spinal cord, and for the prevention of progressive urinary tract deterioration [635]. At birth, the majority of patients have normal UUTs, but up to 60% develop upper tract deterioration due to bladder changes, UTI and /or VUR, if not treated properly [636-639]. Even today in a contemporary series around 50% of the patients are incontinent and 15% have an impaired renal function at the age of 29 years [640]. A systematic review concerning the outcome of adult meningomyelocele patients demonstrated that around 37% (8-85%) are continent, 25% have some degree of renal damage and 1.3% end stage renal failure [641]. The term “continence” is used differently in the reports, and the definition of “always dry” was used in only a quarter of the reports [642]. A recent nationwide survey in USA showed, that less than 50% of the adult spina population reported being continent [643], which demonstrates the need for better consulting and lifelong support.
The most common presentation at birth is myelodysplasia. The incidence of neural tube defects in Europe is 9.1 per 10,000 births and has not decreased in recent years, despite longstanding recommendations concerning folic acid supplementations [644]. The term myelodysplasia includes a group of developmental anomalies that result from defects in neural tube closure. Lesions include spina bifida aperta and occulta, meningocele, lipomyelomeningocele, or myelomeningocele. Myelomeningocele is by far the most common defect seen and the most detrimental.
With antenatal screening spina bifida can be diagnosed before birth with the possibility of intrauterine closure of the defect [645,646]. Traumatic and neoplastic spinal lesions of the cord are less frequent in children but can also cause severe urological problems. Other congenital malformations or acquired diseases can cause a neurogenic bladder, such as total or partial sacral agenesis which can be part of the caudal regression syndrome [647]. In any child presenting with anorectal malformation (ARM) and cloacal malformations, the development of a neurogenic bladder is possible [648]. Patients with cerebral palsy may also present with varying degrees of voiding dysfunction, usually in the form of uninhibited bladder contractions (often due to spasticity of the pelvic floor and sphincter complex) and wetting. Finally, “non-neurogenic neurogenic” bladder dysfunction, such as Hinman or Ochoa syndrome, have been described, in which no neurogenic anomaly can be found, but severe bladder dysfunction as seen in neurogenic bladders is present [649,650].
3.12.2. Classification systems
As bladder sphincter dysfunction is poorly correlated with the type and spinal level of the neurological lesion, urodynamic and functional classifications are much more practical for defining LUT pathology and planning treatment in children.
The bladder and sphincter are two units working in harmony to act as a single functional unit. In patients with a neurogenic disorder, the storage and emptying phase of the bladder function can be disturbed. The bladder and sphincter may be either overactive or underactive and present in four different combinations. This classification system is based on urodynamic findings [651-653]:
- Overactive sphincter and overactive bladder.
- Overactive sphincter and underactive bladder.
- Underactive sphincter and overactive bladder.
- Underactive sphincter and underactive bladder.
3.12.3. Diagnostic evaluation
Today several guidelines and timetables are used [654-656]. The Panel advocate proactive management in children with spinal dysraphism. In those with a safe bladder during the first urodynamic investigation, the next urodynamic investigation can be delayed until one year of age [4].
3.12.3.1. History and clinical evaluation
History should include questions on clean intermittent catheterisation (CIC) frequency, urine leakage, bladder capacity, UTI, medication, bowel function as well as changes in neurological status. A thorough clinical evaluation is mandatory including the external genitalia and the back. A two-day diary, recording drinking volume and times as well as CIC intervals, bladder volume and leakage can provide additional information about the efficacy of the treatment.
3.12.3.2. Laboratory and urinalysis
After the first week of life, plasma creatinine level should be obtained, later in life; cystatin level is more accurate [657,658]. If there is any sign of decreased renal function, physicians should be encouraged to optimize the treatment as much as possible. The criteria for urine analysis are the same as for UTI (refer to Chapter 3.9). However, it is much easier for caregivers or patients to obtain catheter urine in patients who are on CIC. They can also perform a dip stick analysis to screen for UTI at home. (For relevance see Section 3.12.4.5). Albuminuria is an early marker of renal disease also in children with neurogenic bladder [659].
3.12.3.3. Ultrasound
At birth, US of the kidneys and bladder should be performed and then repeated at least annually. If there are any clinical changes in between, another US should be performed. Dilatation of the UUT should be reported according to the classification system of the Society of Foetal Urology [660], including the measurement of caliceal dilatation and anterior posterior diameter of the renal pelvis. Residual urine and bladder wall thickness should also be noted. A dilated ureter behind the bladder should be recorded. Bladder wall thickness has been shown not to be predictive of high pressures in the bladder during voiding and storage and cannot be used as a non-invasive tool to judge the risk for the UUT [661].
3.12.3.4. Urodynamic studies/videourodynamic
Urodynamic studies (UD) are one of the most important diagnostic tools in patients with neurogenic bladders. In newborns with spina bifida aperta, the first UD should be performed after the phase of spinal shock after closure, usually between the second and third months of life [662]. Especially in newborns, performing and interpretation of UD may be difficult, as no normal values exist. After that it should be repeated annually, depending on the clinical situation. During and after puberty bladder capacity, maximum detrusor pressure and detrusor leak point pressure increase significantly [663]. Therefore, during this time, a careful follow-up is mandatory.
3.12.3.4.1. Preparation before urodynamic studies
Before any UD a urine analysis should be undertaken. The first assessment should be done under antibiotic prophylaxis. A Cochrane analysis of nine randomised controlled trials showed, that the administration of prophylactic antibiotics compared to placebo reduced the risk of significant bacteriuria from 12% to 4% after UD. However, this was without significant difference for symptomatic UTI (20% vs. 28%), fever or discomfort [664]. If there is significant bacteriuria, antibacterial treatment should be discussed; especially in older patients a single dose may be sufficient [665].
Generally, UD-parameters should include:
- the cystometric capacity;
- the intravesical filling pressure;
- detrusor compliance;
- the intravesical pressure at the moment of voiding or leakage;
- the presence or absence of detrusor overactivity;
- the competence of the internal and external sphincter;
- the degree of synergy of the detrusor and sphincter during voiding;
- the PVR volume.
In infants, information on detrusor filling pressure and the pressure and bladder volume at which the child voids or leaks can be obtained [662]. Detrusor leak point pressure is more accurate than abdominal leak point pressure but keeping the rectal probe in an infant in place can be challenging [662]. Addition of fluoroscopy (video-urodynamic study) will provide information about presence of VUR, at what pressures VUR occurs and the configuration of the bladder neck during filling and leakage or voiding.
3.12.3.4.2. Uroflowmetry
Unlike in children with non-neurogenic voiding dysfunction, uroflowmetry can rarely be used since most affected patients do not void spontaneously. In those with cerebral palsy, non-neurogenic bladder or other neurological conditions allowing active voiding it may be a practical tool. It provides an objective way of assessing the efficiency of voiding, while recording of pelvic floor activity with electromyography (EMG) can be used to evaluate synergy between detrusor and the sphincter. PVR urine volume is measured by US. The main limitation of uroflowmetry is the compliance of the child to follow instructions [666-669].
3.12.3.5. Urodynamic studies
The standards of the ICCS should be applied to UDs in patients with neurogenic bladders and accordingly reported [540,652]. Natural fill UD in children with neurogenic bladder detects more overactivity compared with diagnoses delivered by conventional UD [670,671]. It may be an option in patients where the findings in the conventional UD are inconsistent with clinical symptoms and other clinical findings [671].
3.12.3.6. Voiding cystourethrogram
If video-urodynamic equipment is not available, a VCUG with UD is an alternative to confirm or exclude VUR and visualise the LUT including the urethra.
3.12.3.7. Renal scan
Technetium Dimercapto-Succinic Acid (DMSA) renal scan is the gold standard to evaluate renal parenchyma. In contemporary series, renal scars can be detected in up to 46% as patients get older [672-674]. In a recent study, 4 out of 68 children had renal scarring, 3 had a history of febrile UTI and one a vesicoureteral reflux [624]. A positive DMSA scan correlates well with hypertension in adulthood, whereas US has a poor correlation with renal scars [674]. Therefore, a DMSA scan as a baseline evaluation in the first year of life is recommended.
3.12.4. Management
The medical care of children with neurogenic bladder requires an on-going multidisciplinary approach. There is some controversy about optimal timing of the management; proactive vs. expectant management [675-677]. Even with a close expectant management e.g. in one series 11 out of 60 need augmentation within a follow-up of 16 years and 7 out of 58 had a decrease in total renal function, which was severe in two [678]. During the treatment it should also be taken into account in spina bifida patients, that QoL is related to urinary incontinence independent of the type and level of spinal dysraphism and the presence or absence of a liquor shunt [679].
Foetal open and endoscopic surgery for meningomyelocele are performed to close the defect as early as possible in order to reduce neurological, orthopaedic and urological problems [680]. The MOMS-Trial found no difference between those closed in utero vs. those closed after birth concerning the need for CIC [646], but less bladder trabeculation was found in the prenatal surgery group. Mean gestation age (28.3 vs. 35.2) seems to have no initial impact on bladder function in the first few years of life [681]. Two European series showed, that there is a possible benefit of open intrauterine closure on urinary continence showing normal bladder function in up to 33% at least in the first 2-3 years of life [682,683]. Despite these promising reports [681,684-686], caregivers need to be aware of the high risk of developing a neurogenic bladder as demonstrated by a Brazilian group [687]. Regular and close follow-up examinations including UD are indicated in all these patients.
3.12.4.1. Early management with intermittent catheterisation
Starting CIC soon after birth and closure of the defect by the neurosurgeon in all infants has shown to decrease renal complications and the need for later augmentation [688-691]. In infants without any clear sign of outlet obstruction, this may be delayed but only in very selected cases. These infants should be monitored very closely for UTIs and changes of the urinary tract with US and UD. The early initiation of CIC in the new-born period makes it easier for caregivers to master the procedure and for children to accept it, as they grow older [692,693]. Up to 90% of patients will perform CIC [694].
A Cochrane review as well as a recent study showed, that there is a lack of evidence to state that the incidence of UTI is affected by use of sterile or clean technique, coated or uncoated catheters, single (sterile) or multiple use (clean) catheters, self-catheterisation or catheterisation by others, or by any other strategy [695-699]. Looking at the microbiological milieu of the catheter, there was a trend for reduced recovery of potentially pathogenic bacteria with the use of hydrophilic catheters. Also, a trend for a higher patient satisfaction with the use of hydrophilic catheters was seen [700]. Based on the current data, it is not possible to state that one catheter type, technique or strategy is better than another.
3.12.4.2. Medical therapy
Antimuscarinic/anticholinergic medication reduces/prevents detrusor overactivity and lowers intravesical pressure [701,702]. Effects and side effects depend on the distribution of the M1-M5 receptors [703]. In the bladder, the subtype M2 and M3 are present [702,704]. Oxybutynin is the most frequently used in children with neurogenic bladder with a success rate of up to 93% [705,706]. Dose-dependent side-effects (such as dry mouth, facial flushing, blurred vision heat intolerance etc.) limit its use. Intravesical administration gives a significant higher bioavailability due to the circumvention of the intestinal first pass metabolism, as well as possible local influence on C-fiber-related activity and can be responsible for different clinical effect [707,708].
Intravesical administration should be considered in patients with severe side-effects, as long-term results demonstrated that it was well-tolerated and effective [709,710]. Transdermal administration also leads to a substantially lower ratio of N-desethyloxybutynin to oxybutynin plasma levels, however, there are treatment-related skin reactions in 12 out of 41 patients [711]. There are some concerns about central anticholinergic adverse effects associated with oxybutynin [712,713]. A double blinded cross-over trial, as well as a case control study, showed no deleterious effect on children’s attention and memory [674,714] . Tolterodine, solifenacine, fesoterodin, trospium chloride and propiverine and their combinations can also be used in children [715-723].
The oral dosage for oxybutynin is up to 0.2 mg/kg [702] given three times daily. The intravesical dosage can be up to 0.7 mg/kg/daily and transdermal 1.3-3.9 mg/daily. The dosage of the other drugs is: Tolterodine
0.5–4 mg/day divided in two doses, Solifenacin 1.25 up to 10 mg per day (single dose), fesoterodine 4-8 mg per day (single dose) Propiverin 0.8 mg/kg/day divided in two dosages and trospium chloride up to 3 times
15 mg starting with 3 times 5 mg. Except for oxybutynin, all other anticholinergic drugs are off-label use, which should be explained to the caregivers.
Early prophylactic treatment with anticholinergics showed a lower rate of renal deterioration as well as a lower rate of progression to bladder augmentation [688,724]. Beta-3 agonists like mirabegron as an adjuvant treatment has been shown to be effective and safe in some recent studies of children (> five years) and adolescents [725-728].
Alpha-adrenergic antagonists may facilitate emptying in children with neurogenic bladder [729]. Doxazosin with an initial dose of 0.5 to 1.0 mg or tamsulosin hydrochloride in a medium (0.0002-0.0004 mg/kg/day) or high dose (0.0004-0,0008 mg/kg/day) has been given to children with neurogenic bladders [729-731]. It was well tolerated but not effective at least in one study [730].
Botulinum toxin A injections: In neurogenic bladders that are refractory to anticholinergics, the off-label use of suburothelial or intramuscular injection of onabotulinum toxin A into the detrusor muscle is a treatment option [653,654]. In children, continence could be achieved in 32-100% of patients, a decrease in maximum detrusor pressure of 32-54%, an increase of maximum cystometric capacity from 27-162%, and an improvement in bladder compliance of 28-176% [653]. Onabotulinum toxin A seems to be more effective in bladders with obvious detrusor muscle over-activity, whereas non-compliant bladders without obvious contractions are unlikely to respond [732,733]. Also, the injections into the trigone seems to be save in regard of reflux and upper tract damage; if it has some benefit is not further investigated [657]. Of the patients with failed augmentation cystoplasty, 43% responded well to intra-detrusor onabotulinum toxin A injections in a recent series of 30 patients [734].
The most used dose of onabotulinum toxin A is 10 to 12 U/kg with a maximum dose between 200 U and
360 U [735]. A recent RCT demonstrated, that 200 IE have greater efficacy in reducing bladder pressure and increasing bladder capacity compared to 50 or 100 IE [736]. Onabotolinum toxin A can be effective for between three to twelve (0-25) months and repeated injections were effective for up to ten years in one study [737-739].
Urethral sphincter onabotulinum toxin A injection has been shown to be effective in decreasing urethral resistance and improve voiding. The evidence is still too low to recommend its routine use in decreasing outlet resistance, but it could be considered as an alternative in refractory cases [740,741].
Neuromodulation
Intravesical electrical stimulation of the bladder [742-744], sacral nerve stimulation [745,746] and transcutaneous neuromodulation [668] are still experimental and cannot be recommended outside of clinical trials. The same is true for the intradural somatic-to-autonomic nerve anastomosis [747,748].
Urethral Dilatation
The aim is to lower the pop-off pressure by lowering the detrusor leak-point pressure by dilatation of the external sphincter under general anaesthesia up to 36 Charr. Some studies showed, that especially in females, the procedure is safe and in selected patients, effective [749-751].
Vesicostomy
Vesicostomy - preferably a Blocksom stoma [752] - is an option to reduce bladder pressure in children/new-borns, if the caregivers are incompliant with CIC and/or CIC through the urethra is extremely difficult or impossible [753]. Especially in the young infant with severe upper tract dilatation or infections, a vesicostomy should be considered. In some patients it may be also a good long-term solution to prevent infection and renal deterioration [754]. Drawbacks are the difficulty fitting and maintaining a collecting appliance in older patients. A cystostomy button may be an alternative, with a complication rate (mostly UTI) of up to 34% within a mean follow-up of 37 months [755].
3.12.4.3. Management of faecal incontinence
Children with neurogenic bladder usually have also a neurogenic bowel function. Faecal incontinence may have an even greater impact on QoL, as the odour can be a reason for social isolation. The aim of each treatment is to obtain a smooth, regular bowel emptying and to achieve continence and impendence. The regime should be tailored to the patient’s need, which may change over time. Beside a diet with small portioned fibre food and adequate fluid intake to keep a good fluid balance [702], follow-up options should be offered to the patients and caregivers.
In the beginning, faecal incontinence is managed most commonly with mild laxatives, such as mineral oil, combined with enemas to facilitate removal of bowel contents. To enable the child to defecate once a day at a given time, rectal suppositories as well as digital stimulation by parents or caregivers can be used. Today, transanal irrigation is one of the most important treatments for patients with neurogenic bowel incontinence. Regular irrigations significantly reduce the risk for faecal incontinence also in the long run in up to 90% of the patients [756]. The risk of irrigation induced perforation of the bowel is estimated as one per 50,000 [678]. During childhood, most children depend on the help of the caregivers. Later in some patients, transanal irrigation becomes difficult or impossible due to anatomic or social circumstances. In these patients antegrade irrigation using a MACE-stoma (Malone Antegrade Continence Enema) is an option, which can also be placed in the left abdomen [757,758]. In a long-term study of 105 patients with a MACE stoma, 69% had successful bowel management. They were started on normal saline, but some switched to GoLYTELY (PEG-3350 and electrolyte solution). Additives (biscodyl, glycerin etc.) were needed in 34% of patients. Stomal complications occurred in 63% (infection, leakage, and stenosis) of patients, 33% required surgical revision and 6% eventually required diverting ostomies [759]. In addition, patients need to be informed, that the antegrade irrigation is also time consuming taking at least 20-60 minutes.
3.12.4.4. Urinary tract infection
Urinary tract infections are common in children with neurogenic bladders. However, there is no consensus in most European centres, for prevention, diagnosing and treating UTIs in children with neurogenic bladders performing CIC [760]. Although bacteriuria is seen in more than half of children on CIC, patients who are asymptomatic do not need treatment [761]. Continuous antibiotic prophylaxis (CAP) creates more bacterial resistance as demonstrated by a randomized study. Those that stopped the prophylaxis had reduced bacterial resistance, however, 38 out of 88 started antibiotic prophylaxis again due to recurrent UTIs or the caregivers request [762]. A cohort study with 20 patients confirmed these findings. Continuous antibiotic prophylaxis was not protective against the development of symptomatic UTIs and new renal scarring but increased the risk of bacterial resistance [763]. A randomized study in 20 children showed that cranberry capsules significantly reduced the UTI-rate as well as the rate of bacteriuria [764]. If VUR is present, prophylactic antibiotics should be started when patients experience recurrent UTIs [765,766].
3.12.4.4.1. Urinary tract infection and clean intermittent catheterisation
The incidence of asymptomatic bacteriuria ranges between 42-76% [692,702,767]. A cross-over study in 40 children with neurogenic bladder demonstrated, that the reuse of CIC-catheters for up to three weeks compared to one week increased the prevalence of bacteriuria from 34-74% (it was 60% at the start of the study). During the study-period of eighteen weeks, none of the patients developed a febrile UTI [768]. There is no medical benefit in performing CAP in children with neurogenic bladder, who perform CIC [702]. In those with recurrent UTI, intravesical instillation of gentamycin or neomycin/polymyxin may be an option [769,770].
Reflux
Secondary reflux in patients with neurogenic bladder increases the risk for pyelonephritis. The treatment is primary related to bladder function including anticholinergic therapy, CIC and may be later augmentation [771]. Those with early and post-therapy persistent reflux during videourodynamic studies at low pressure have a higher risk of pyelonephritis [772]. Patients with a high-grade reflux before augmentation have a higher risk of persistent symptomatic reflux after the enterocystoplasty [773]. Therefore simultaneous ureteral re-implantation in high-grade symptomatic reflux especially in those with low-pressure high-grade reflux should be discussed with the patient/caregivers. Endoscopic treatment has a failure rate of up to 75% after a median follow-up of 4.5 years [774] which is in contrast to the open techniques with a higher success rate but may have an increased risk of inducing obstruction [775].
3.12.4.5. Sexuality
Sexuality, while not an issue in childhood, becomes progressively more important as the patient gets older. This issue has historically been overlooked in individuals with myelodysplasia. However, patients with myelodysplasia do have sexual encounters [776]. The prevalence of precocious puberty is higher in girls with meningomyelocele [777]. Studies indicate that at least 15-20% of males are capable of fathering children and 70% of females can conceive and carry a pregnancy to term. It is therefore important to counsel patients about sexual development in early adolescence.
Women seem to be more sexually active than men in some studies from the Netherlands and the USA [776,778]. The level of the lesion was the main predictor to be sexually active [779,780]. Erectile function can be improved by sildenafil in up to 80% of the male patients [781,782] . Neurosurgical anastomosis between the inguinal nerve and the dorsal penile nerve in patients with a lesion below L3 and disturbed sensation is still to be considered as an experimental treatment [778,783]. Only 17% to one third of the patients talk to their doctors about sexuality, 25–68% were informed by their doctors about reproductive function [776]. Continence seems to play an important role too. Nine out eleven females without sexual dysfunction reported continence, whereas 50 out of 59 with sexual dysfunction have some urinary incontinence in a recent study [784]. Therefore, early discussion about sexuality in the adolescent is recommended and should be promoted by the paediatric urologist taking care of these patients.
3.12.4.6. Bladder augmentation
In patients where conservative treatment including onabotulinum toxin A (for indication see 3.12.4.3) fails to keep a low-pressure reservoir with a good capacity and compliance, bladder augmentation should be offered. For augmentation, ileal and colonic segments can be used [785]. Gastric segments are rarely used due to its associated complications like the haematuria-dysuria syndrome as well as secondary malignancies, which arise earlier than with other intestinal segments [786-789]. Enterocystoplasty increases bladder capacity, reduces storage pressure and can improve UUT drainage [790]. A good socially acceptable continence rate can be achieved with or without additional bladder outlet procedures [791]. In those, who are not able to perform CIC through the urethra, a continent cutaneous channel should be offered. One recent study in 10 patients showed that thoracic epidural analgesia appears to be a safe and effective opioid sparing option to assist with postoperative pain management following LUT reconstruction [792]. Surgical complications and revision rate in this group of patients is high. The 30-day all over event rate in the American College of Surgeons’ National Surgical Quality Database is approximately 30% (23-33%) with a re-operation rate in this short time period of 13% [793,794]. In these patients with long-life expectancy the complication rate clearly increases with the follow-up period [795]. The ten-year cumulative complication incidence from the Paediatric Health Information System showed a rate of bladder rupture in up to 6.4%, small bowel obstruction in up to 10.3%, bladder stones in 36%, pyelonephritis in more than a third of the patients and a re-augmentation rate of up to 13% [796]. Bladder perforation, as one of the worst complications, occurs in 3-13% [797]. The rate of VP-shunt infections after gastrointestinal and urological procedures ranges between 0-22%. In a recent study, bowel preparation seems not to have a significant influence on the infection rate (10.5% vs. 8.3%) [798]. Not only surgical complications must be considered; also metabolic complications and consequences after incorporating bowel segments have to be taken into account, such as imbalance of the acid base balance, decrease in vitamin B12 levels and loss of bone density. Stool frequency can increase as well as diarrhoea after exclusion of bowel segments [799] and last, but not least, these patients have a lifelong increased risk to develop secondary malignancies [800,801]. Therefore, a lifelong follow-up of these patients is required including physical examination, US, blood gas analysis, (pH and base excess), renal function and vitamin B12 if Ileum is used. Endoscopic evaluation starting ten years after augmentation is not cost-effective [802,803], but may prevent some advanced cancer. Woodhouse et al. do not recommend cystoscopy within the first fifteen years after surgery [804]. The real value of annual cystoscopic evaluation has not been proven by any study. Urodynamic studies after bladder augmentation are only indicated, if upper tract dilatation and/or incontinence after the operation has not improved [805].
Adverse effects of intestinal cystoplasties can be avoided by the use of ureterocystoplasty. The combination of a small contracted bladder, associated with a severe dilation of the ureter of a non-functioning kidney is quite rare. The technique was first described in 1973 by Eckstein [806]; the success rate depends on patient selection and the re-augmentation rate can reach 73% [807,808].
Auto-augmentation with partial detrusorectomy or detrusormyotomy creating a diverticulum avoids metabolic complications with the use of intestinal segments. The reports are conflicting, therefore, it may be used in selected cases [809-812]. For a successful outcome, a pre-operative bladder capacity of 75-80% of the expected volume seems necessary [810,813]. Seromuscular cystoplasty has also not proven to be as successful as standard augmentation with intestine [814]. Tissue engineering, even if successful in vitro and some animal models, does not reach the results by using intestinal segments with a higher complication rate [815,816]. Therefore, these alternatives for bladder augmentation should be considered as experimental and should be used only in controlled trials.
3.12.4.7. Bladder outlet procedures
No available medical treatment has been validated to increase bladder outlet resistance. Alpha-adrenergic receptor stimulation of the bladder neck has not been effective [729]. Using fascial slings with autologous fascial strip or artificial material a continence rate between 40-100% can be achieved. In most cases this is achieved in combination with bladder augmentation [817-822]. Catheterising through a reconstructed bladder neck or a urethra compressed by a sling may not be easy; many surgeons prefer to combine this approach with a catheterisable channel [675]. In contrast to the autologous slings, artificial slings in girls with CIC through the urethra have a high complication rate [823]. In males, it may be an option [824], however as long as long-term results are missing, this method has to be classified as experimental and should only be carried out in studies. Artificial urinary sphincters were introduced by Scott in 1973 [825]. The continence rates in the literature in selected patients can be up to 83% [826,827]. Post-pubertal patients, who can void voluntary are good candidates, if they are manually dexterous. In very selected patients, CIC through the sphincter in an augmented bladder is possible [827]. The erosion rate can be up to 29% and the revision rate up to 100% depending on the follow-up time [821].
Patients, who underwent a bladder neck procedure only, have a chance of > 30% for an augmentation and/or onabotulinum toxin A injections > 30% later on; half of them developed new upper tract damage in that time [828-830]. In patients with a good bladder capacity and bladder compliance without an indication for bladder augmentation, up to 40% will need augmentation later on [829]. Therefore, close follow-up of these patients with UD is required to avoid upper tract damage and chronic renal failure.
Bladder neck reconstruction is used mostly in exstrophy patients with acceptable results. However, in children with a neurogenic bladder the results are less favourable [831]. In most patients, the creation of a continent catheterisable stoma is necessary due to difficulties in performing the CIC via the urethra. In one series, 10% to a third still performed CIC via the urethra with a re-operation rates between 67% and 79% after a median follow-up between seven and ten years [832]. In patients who are still incontinent after a bladder outlet procedure, bladder neck closure with a continent catheterisable stoma is an option. The combination of a sling procedure together with a urethral lengthening procedure may improve the continence rates [833].
Bulking agents have a low success rate (10-40%), which is in most cases only temporary [834-836]. However, it does not adversely affect the outcome of further definite surgical procedures [834].
Bladder neck closure is often seen as the last resort to gain urinary continence in those patients with persistent urinary incontinence through the urethra. In girls, the transection is done between bladder neck and urethra and in boys above the prostate with preservation of the neurovascular bundle. It is an effective method to achieve continence together with a catheterisable cutaneous channel +/- augmentation as a primary or secondary procedure [837,838]. A complication rate of up to a third and a vesicourethral/vesicovaginal fistula in up to 15% should be considered [839], together with a higher risk for bladder stones, bladder perforation and deterioration of the upper tract function, if the patient is not compliant with CIC and bladder irrigations [839,840].
3.12.4.8. Catheterisable cutaneous channel.
In most patients with a neurogenic bladder CIC is required. If this is not possible, or very time and/or resource consuming via the urethra, a continent cutaneous catheterisable channel should be offered as well as in those with bladder outlet procedures. It is especially beneficial to wheelchair-bound patients who often have difficulty with urethral catheterisation or are dependent on others to catheterise the bladder. In long-term studies the revision rate due to stenosis or incontinence can be as high as 50-60% depending on the type of channel [841,842].
The stoma can be placed at the umbilicus or in the lower right abdominal wall using a VQZ plasty [843]. It should be carefully evaluated pre-operatively: it is extremely important that the patient can reach the stoma easily. Sometimes it has to be placed in the upper abdominal wall due to sever scoliosis mostly associated with obesity.
3.12.4.9. Continent and incontinent cutaneous urinary diversion
Incontinent urinary diversion should be considered in patients who are not willing or able to perform a CIC and who need urinary diversion because of upper tract deterioration or gain urinary continence due to social reasons. In children and adolescents, the colonic conduit has shown to have less complications compared to the ileal conduit [844-847]. Total bladder replacement is extremely rare in children and adolescents, but may be necessary in some adults due to secondary malignancies or complications with urinary diversions. Any type of major bladder and bladder outlet construction should be performed in centres with sufficient experience in the surgical technique, and with experienced healthcare personnel to carry out post-operative follow-up [791,848,849].
Algorithms can be used for management of these patients (Figures 7 and 8).
3.12.5. Follow-up
Neurogenic bladder patients require lifelong follow-up including not only urological aspects but also neurological and orthopaedic aspects. Regular investigation of upper and lower urinary tract is mandatory. In patients with changes of the function of the upper and/or lower urinary tract, a complete neurological re-investigation should be recommended including a total spine MRI to exclude a secondary tethered cord or worsening of the hydrocephalus. In addition, if some neurological changes are observed a complete investigation of the urinary tract should be undertaken.
A recent study of this guideline panel revealed that the priorities of patients for future expectations were as following in decreasing order: GoL, surgical techniques, development of new medications and sexuality/fertility issues. Male spina bifida patients preferred new medications and sex/fertility issues more, whereas females favoured QoL issues improvement more. These factors should be considered during long-term management [2].
In those patients with urinary tract reconstruction using bowel segments, regular investigations concerning renal function, acid base balance and vitamin B12 status are mandatory to avoid metabolic complications. There is an increased risk for secondary malignancies in patients with a neurogenic bladder either with or without enteric bladder augmentations [850-854]. Therefore, patients need to be informed of this risk and possible signs like haematuria. Although there are insufficient data on follow-up schemes to discover secondary malignancies, after a reasonable follow-up time (e.g. ten to fifteen years), an annual cystoscopy can be considered.
3.12.6. Self-organisation of patients
As patients’ self-organisations can support the parents, caregivers and the patients in all aspects of their daily life, patients should be encouraged to join these organisations.
Figure 7a: Management of children with myelodysplasia with a neurogenic bladderFlowchart - First year of life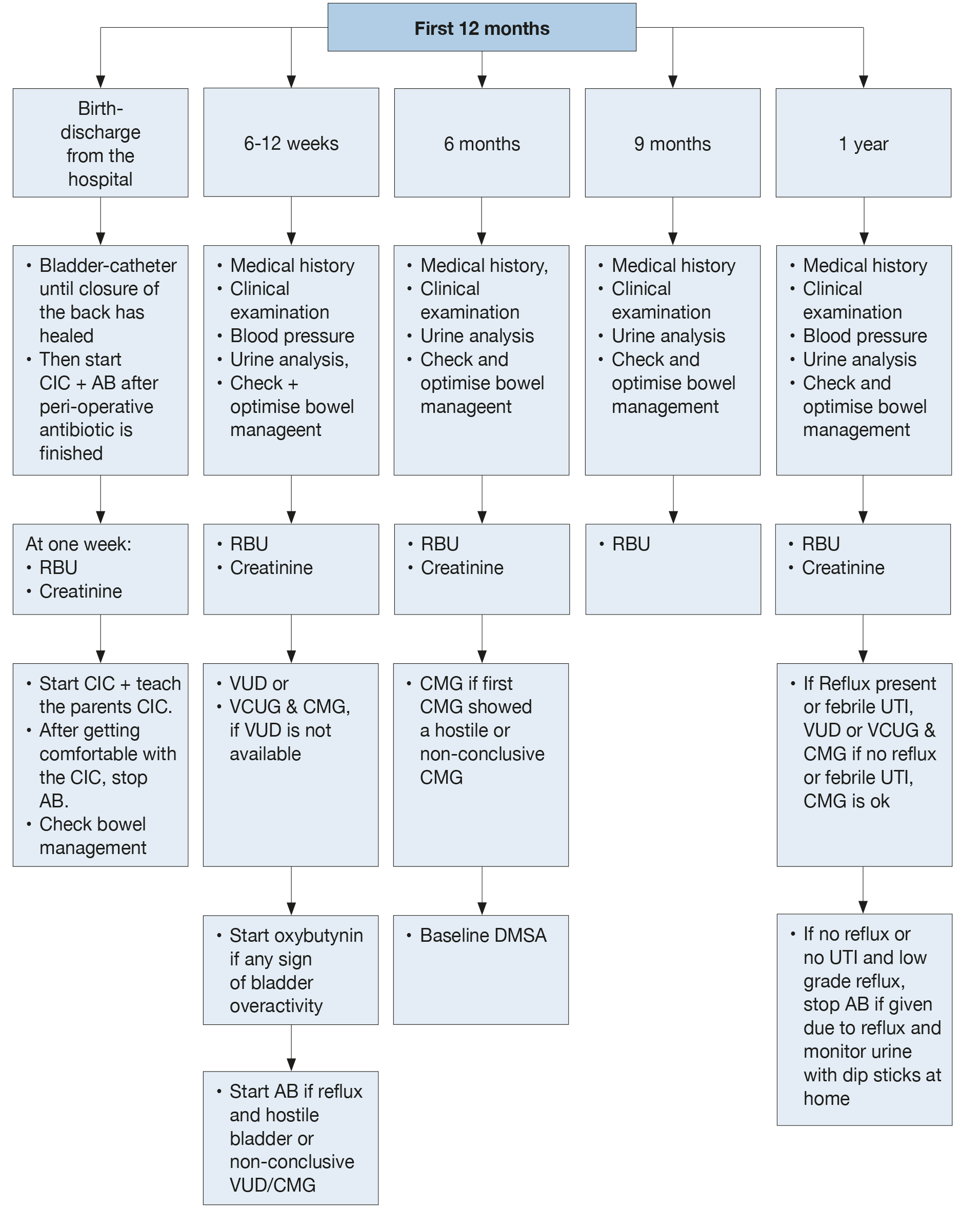 RBUS = Renal bladder ultrasound; UTI = urinary tract infection; VUD = videourodynamic;VCUG = voiding cystourethrography; CIC = clean intermittent catheterisation; CMG = cystometrogram; DMSA = dimercaptosuccinic acid; AB = antibiotics.
RBUS = Renal bladder ultrasound; UTI = urinary tract infection; VUD = videourodynamic;VCUG = voiding cystourethrography; CIC = clean intermittent catheterisation; CMG = cystometrogram; DMSA = dimercaptosuccinic acid; AB = antibiotics.
Figure 7b: Management of children with myelodysplasia with a neurogenic bladder Flowchart - 18 months - 4 years of age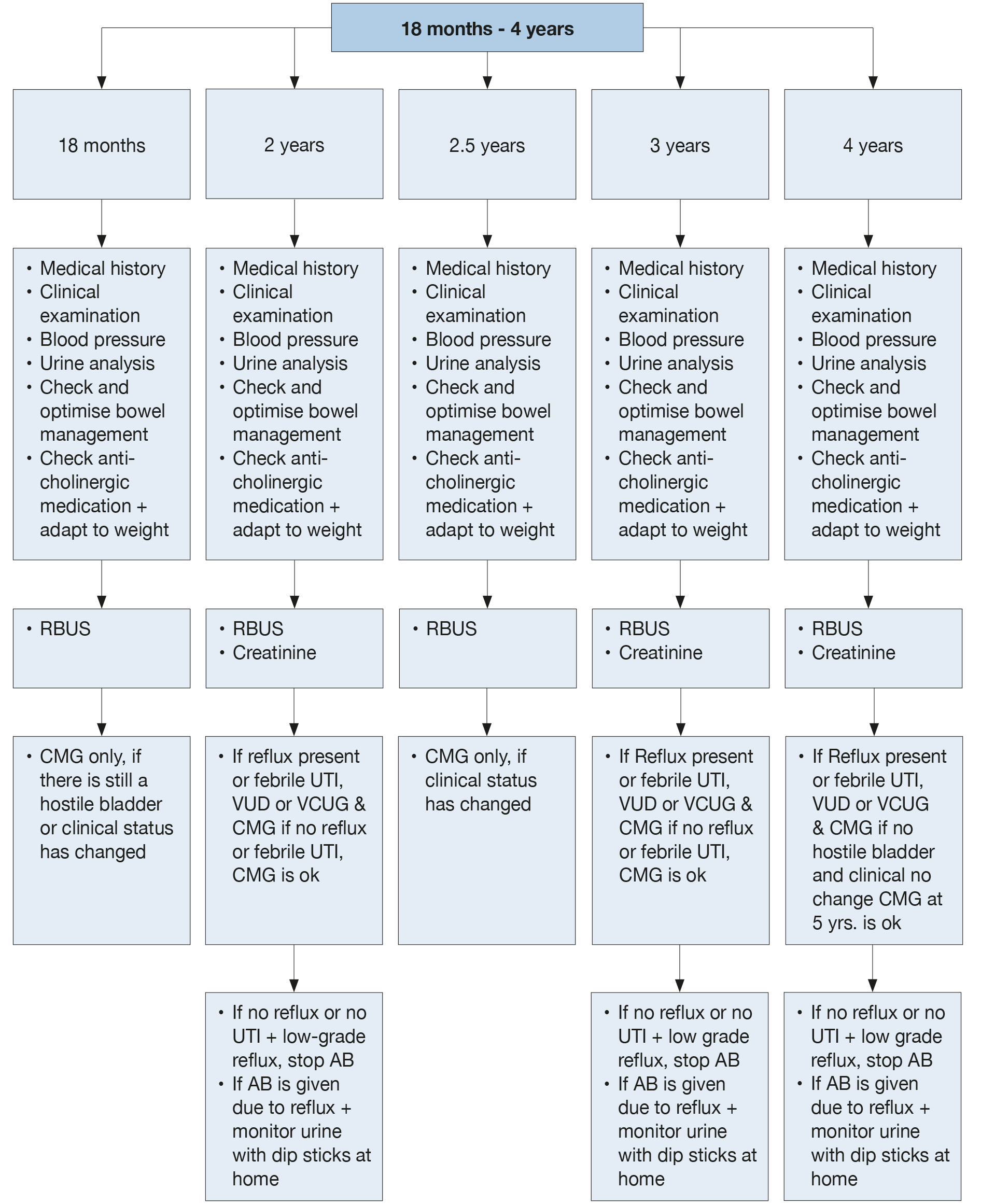 RBUS = Renal bladder ultrasound; UTI = urinary tract infection; VUD = videourodynamic;VCUG = voiding cystourethrography; CMG = cystometrogram; DMSA = dimercaptosuccinic acid; AB = antibiotics
RBUS = Renal bladder ultrasound; UTI = urinary tract infection; VUD = videourodynamic;VCUG = voiding cystourethrography; CMG = cystometrogram; DMSA = dimercaptosuccinic acid; AB = antibiotics
Figure 7c: Management of children with myelodysplasia with a neurogenic bladder Flowchart - 5 years to adulthood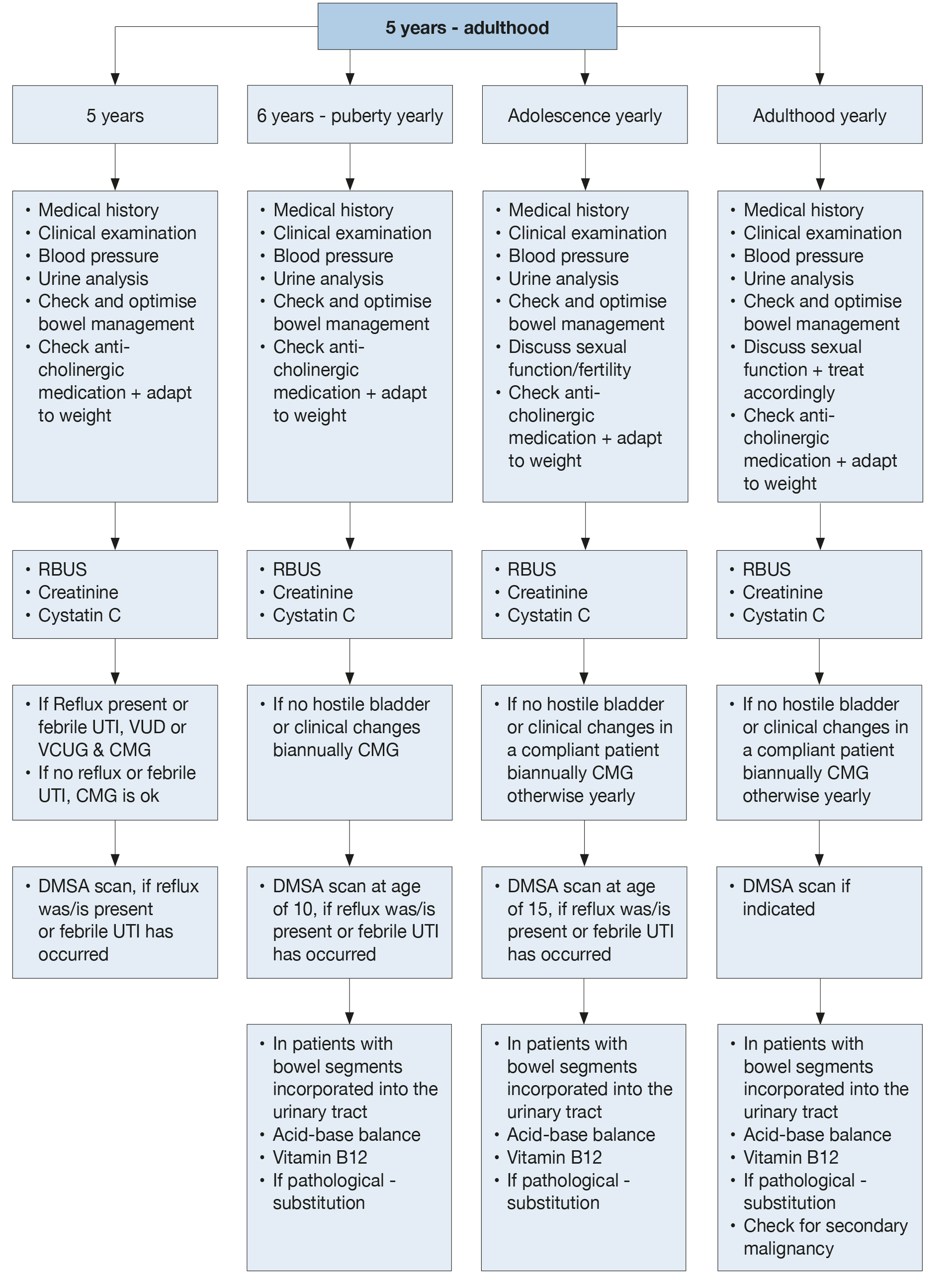 RBUS = Renal bladder ultrasound; UTI = urinary tract infection; VUD = videourodynamic;VCUG = voiding cystourethrography; CMG = cystometrogram; DMSA = dimercaptosuccinic acid.
RBUS = Renal bladder ultrasound; UTI = urinary tract infection; VUD = videourodynamic;VCUG = voiding cystourethrography; CMG = cystometrogram; DMSA = dimercaptosuccinic acid.
Figure 8: Algorithm for the management of children with myelodysplasia with a neurogenic bladder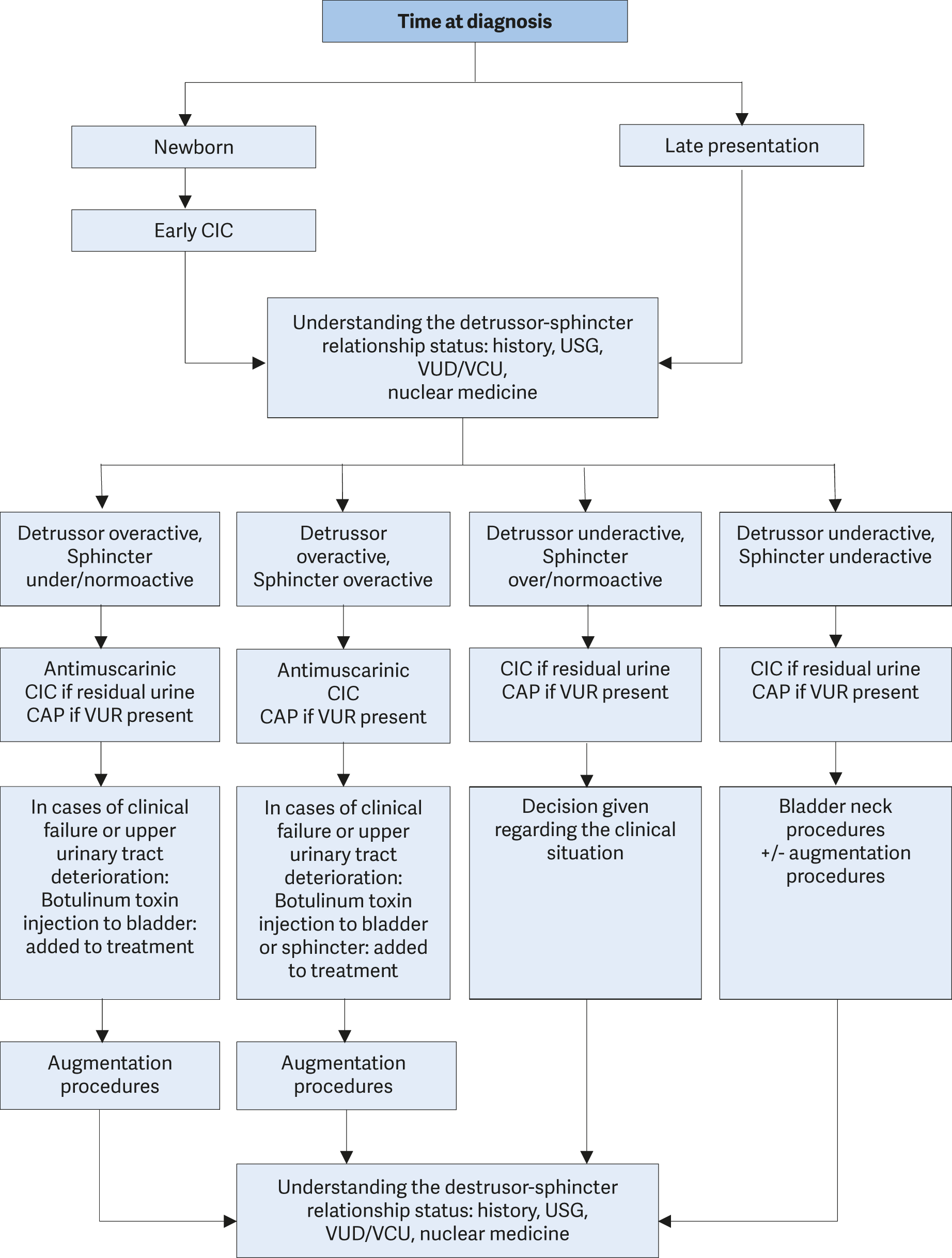 CAP = continuous antibiotic prophylaxis; CIC = clean intermittent catheterisation; US = ultrasound;VCUG = voiding cystourethrography; VUD = videourodynamic; VUR = vesicoureteric reflux.
CAP = continuous antibiotic prophylaxis; CIC = clean intermittent catheterisation; US = ultrasound;VCUG = voiding cystourethrography; VUD = videourodynamic; VUR = vesicoureteric reflux.
3.12.7. Summary of evidence and recommendations for the management of neurogenic bladder
Summary of evidence | LE |
Neurogenic detrusor-sphincter dysfunction (NDSD) may result in different forms of LUTD and ultimately result in incontinence, UTIs, VUR, and renal scarring. | 2a |
In children, the most common cause of NDSD is myelodysplasia (a group of developmental anomalies that result from defects in neural tube closure). | 2 |
Bladder sphincter dysfunction correlates poorly with the type and level of the spinal cord lesion. Therefore, urodynamic and functional classifications are more practical in defining the extent of the pathology and in guiding treatment planning. | 2a |
Children with neurogenic bladder can have disturbances of bowel function as well as urinary function which require monitoring and, if needed, management. | 2a |
The main goals of treatment are prevention of urinary tract deterioration and achievement of continence at an appropriate age. | 2a |
Injection of botulinum toxin into the detrusor muscle in children who are refractory to anticholinergics, has been shown to have beneficial effects on clinical and urodynamic variables. | 2a |
Recommendations | LE | Strength rating |
Urodynamic studies should be performed in every patient with spina bifida as well as in every child with high suspicion of a neurogenic bladder to estimate the risk for the upper urinary tract and to evaluate the function of the detrusor and the sphincter. | 2 | Strong |
In all newborns, intermittent catheterisation (IC) should be started soon after birth. In those with a clear underactive sphincter and no overactivity, starting IC may be delayed. If IC is delayed, closely monitor babies for urinary tract infections, upper tract changes (ultrasound) and the lower tract (urodynamics). | 3 | Strong |
Start early anticholinergic medication in the newborns with suspicion of an overactive detrusor. | 2 | Strong |
The use of suburothelial or intradetrusoral injection of onabotulinum toxin A is an alternative and a less invasive option in children who are refractory to anticholinergics in contrast to bladder augmentation. | 2 | Strong |
Treatment of faecal incontinence is important to gain continence and independence. Treatment should be started with mild laxatives, rectal suppositories as well as digital stimulation. If not sufficient transanal irrigation is recommended, if not practicable or feasible, a Malone antegrade colonic enema (MACE)/Antegrade continence enema (ACE) stoma should be discussed. | 3 | Strong |
Ileal or colonic bladder augmentation is recommended in patients with therapy resistant overactivity of the detrusor, small capacity and poor compliance, which may cause upper tract damage and incontinence. The risk of surgical and non-surgical complications and consequences outweigh the risk of permanent damage of the upper urinary tract +/- incontinence due to the detrusor. | 2 | Strong |
In patients with a neurogenic bladder and a weak sphincter, a bladder outlet procedure should be offered. It should be done in most patients together with a bladder augmentation. | 3 | Weak |
Creation of a continent cutaneous catheterisable channel should be offered to patients who have difficulties in performing an IC through the urethra. | 3 | Weak |
A life-long follow-up of renal and reservoir function should be available and offered to every patient. Addressing sexuality and fertility starting before/during puberty should be offered. | 3 | Weak |
Urinary tract infections (UTIs) are common in children with neurogenic bladders, however, only symptomatic UTIs should be treated. | 3 | Weak |
3.13. Dilatation of the upper urinary tract (UPJ and UVJ obstruction)
3.13.1. Epidemiology, aetiology and pathophysiology
Dilatation of the upper urinary tract (UUT) remains a significant clinical challenge in deciding which patient will benefit from treatment. Ureteropelvic junction (UPJ) obstruction is defined as impaired urine flow from the pelvis into the proximal ureter with subsequent dilatation of the collecting system and the potential to damage the kidney. It is the most common pathological cause of neonatal hydronephrosis [855]. It has an overall incidence of 1:1,500 and a ratio of males to females of 2:1 in newborns.
Ureterovesical junction (UVJ) obstruction is an obstructive condition of the distal ureter as it enters the bladder, commonly called a primary obstructive megaureter. Megaureters are the second most likely cause of pathological neonatal hydronephrosis. They occur more often in males and are more likely to occur on the left side [856]. It can be very difficult to define ‘obstruction’ as there is no clear division between ‘obstructed’ and ‘non-obstructed’ urinary tracts. Currently, the most popular definition is that an obstruction represents any restriction to urinary outflow that, if left untreated, will cause progressive renal deterioration [857].
3.13.2. Diagnostic evaluation
The widespread use of US during pregnancy has resulted in a higher detection rate for antenatal hydronephrosis (ANH) [858]. The challenge in the management of dilated UUT is to decide which child should be observed, which should be managed medically, and which requires surgical intervention. Despite the wide range of diagnostic tests, there is no single test that can accurately distinguish obstructive from non-obstructive cases (see Figure 9).
3.13.2.1. Antenatal ultrasound
Usually between the 16th and 18th weeks of pregnancy, the kidneys are visualised routinely, when almost all amniotic fluid consists of urine. The most sensitive time for foetal urinary tract evaluation is the 28th week. If dilatation is detected, US should focus on:
- laterality, severity of dilatation, and echogenicity of the kidneys;
- hydronephrosis or hydro-ureteronephrosis;
- bladder volume and bladder emptying;
- sex of the child;
- amniotic fluid volume [859].
3.13.2.2. Postnatal ultrasound
Since transitory neonatal dehydration lasts about 48 hours after birth, imaging should be performed following this period of postnatal oliguria. However, in severe cases (bilateral dilatation, solitary kidney, oligohydramnios), immediate postnatal sonography is recommended [860]. Ultrasound should assess the anteroposterior diameter of the renal pelvis, calyceal dilatation, kidney size, thickness of the parenchyma, cortical echogenicity, ureters, bladder wall and residual urine.
3.13.2.3. Voiding cystourethrogram
In newborns with identified UUT dilatation, the primary or important associated factors that must be detected include:
- vesicoureteral reflux (found in up to 25% of affected children) [861];
- urethral valves;
- ureteroceles;
- diverticula;
- neurogenic bladder.
Conventional VCUG is the method of choice for primary diagnostic procedures [862].
3.13.2.4. Diuretic renography
Diuretic renography is the most commonly used diagnostic tool to detect the severity and functional significance of problems with urine transport. Technetium-99m (99mTc) mercaptoacetyltriglycine (MAG3) is the radionuclide of choice. It is important to perform the study under standardised circumstances (hydration, transurethral catheter) after the fourth and sixth weeks of life [863]. Oral fluid intake is encouraged prior to the examination. At fifteen minutes before the injection of the radionuclide, it is mandatory to administer normal saline intravenous infusion at a rate of 15 mL/kg over 30 minutes, with a subsequent maintenance rate of
4 mL/kg/h throughout the entire time of the investigation [864]. The recommended dose of furosemide is
1 mg/kg for infants during the first year of life, while 0.5 mg/kg should be given to children aged one to sixteen years, up to a maximum dose of 40 mg.
Figure 9: Diagnostic algorithm for dilatation of the upper urinary tract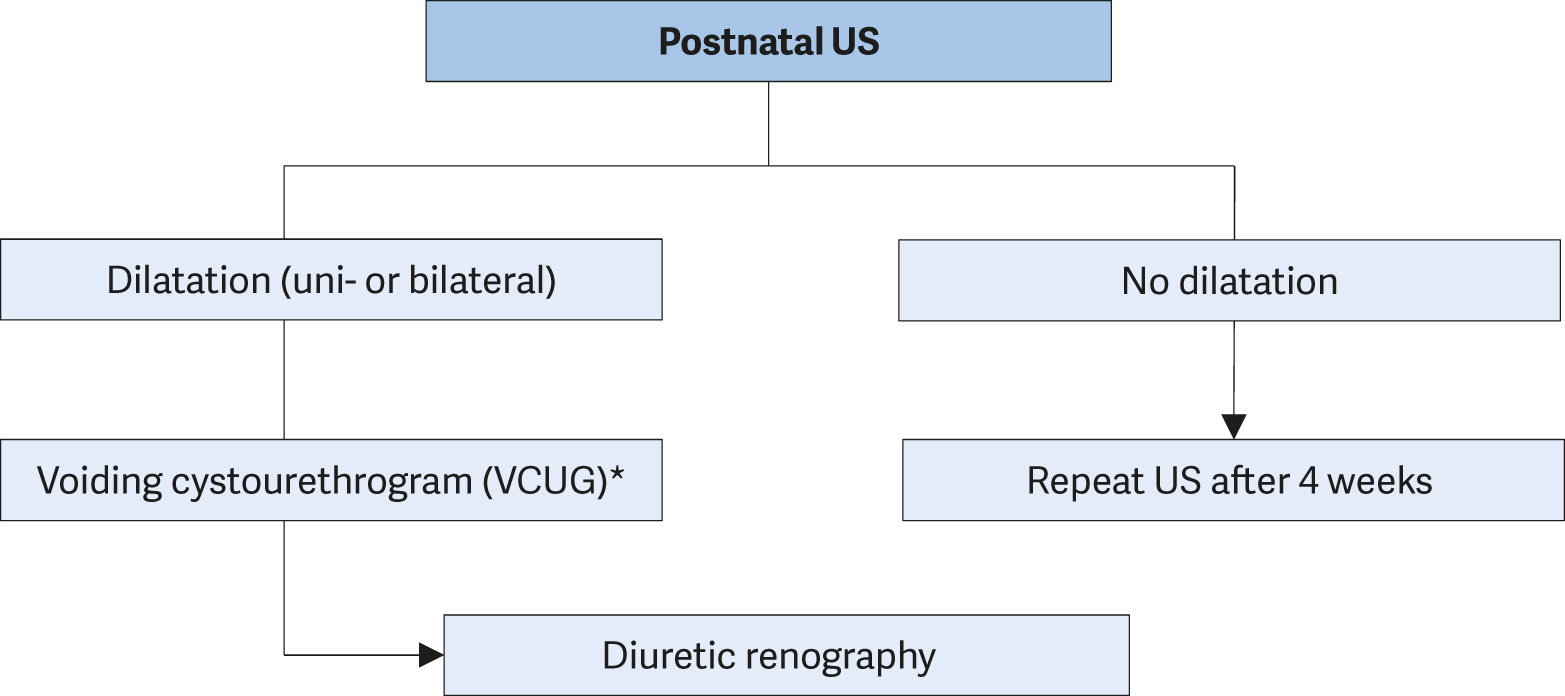 * A diagnostic work-up including VCUG must be discussed with the caregivers, as it is possible that, even if reflux is detected, it may have absolutely no clinical impact. However, it should be borne in mind that reflux has been detected in up to 25% of cases of prenatally detected and postnatally confirmed hydronephrosis .[772] US = ultrasound.
* A diagnostic work-up including VCUG must be discussed with the caregivers, as it is possible that, even if reflux is detected, it may have absolutely no clinical impact. However, it should be borne in mind that reflux has been detected in up to 25% of cases of prenatally detected and postnatally confirmed hydronephrosis .[772] US = ultrasound.
3.13.3. Management
3.13.3.1. Prenatal management
Counselling the caregivers of an affected child is one of the most important aspects of care. The prognosis is hopeful for a hydronephrotic kidney, even if it is severely affected, as it may still be capable of meaningful renal function, unlike a severely hypoplastic and dysplastic kidney.
It is important to be able to tell the caregivers exactly when they will have a definitive diagnosis for their child and what this diagnosis will mean. In some cases, however, it will be immediately obvious that the child is severely affected; there will be evidence of massive bilateral dilatation, bilateral hypoplastic dysplasia, progressive bilateral dilatation with oligohydramnios, and pulmonary hypoplasia.
Intrauterine intervention is rarely indicated and should only be performed in well-experienced centres [865].
3.13.3.1.1. Antibiotic prophylaxis for antenatal hydronephrosis
The benefits and harms of continous antibiotic prophylaxis (CAP) vs. observation in patients with ANH are controversial. Currently, only two RCTs have been published, one of which is a pilot trial [866] and the other publication is only available as a congress abstract [867]. Both publications present incomplete data and outcomes.
The Panel conducted a systematic review assessing the literature from 1980 onwards [868]. The key findings are summarised below.
Due to the heterogeneity of the published literature it was not possible to draw strong conclusions as to whether CAP is superior to observation alone in children diagnosed with antibiotic prophylaxis for antenatal hydronephrosis (ANH). In the first RCT, a prospective longitudinal study [866], female gender, uncircumcised males, lack of CAP, high-grade hydronephrosis, hydroureteronephrosis and VUR were found to be the independent predictors for the development of UTI. The second RCT included in the SR, was published as an abstract only, presented limited data [867]. This trial seemed to focus mainly on patients with ANH and VUR and did not report any beneficial effect of CAP on UTI rates, but details on the study population were limited.
Key findings of the systematic review are that CAP may or may not be superior to observation in children with antenatal hydronephrosis in terms of decreasing UTI. Due to the low data quality it was also not possible to establish whether boys or girls are at a greater risk of developing a UTI, or ascertain whether the presence or absence of VUR impacts UTI rates. A correlation between VUR-grade and UTI could not be established either. However, noncircumcised infants, children diagnosed with high-grade hydronephrosis and hydroureteronephrosis were shown to be at higher risk of developing a UTI.
The SR also tried to identify the most effective antibiotic regimen and present data on adverse effects but, due to heterogeneity, the available data could not be statistically compared. The most commonly used antibiotic in infants with antenatal hydronephrosis is trimethoprim, but only one study reported side effects [866].
In conclusion, based on the available evidence, the benefits and harms of CAP in children with antenatal hydronephrosis remain unproven. Uncircumcised infants and infants with hydroureteronephrosis and highgrade hydronephrosis are more likely to develop a UTI. Continuous antibiotic prophylaxis should be reserved for this sub-group of children who are proven to be at high risk.
3.13.3.2. UPJ obstruction
It is most important that management decisions are made on the basis of serial investigations that have used the same technique and have been performed by the same institution under standardised circumstances. According to a Cochrane review, non-surgical management of unilateral UPJ obstruction in infants less than two years old is also an option. However the high risk of bias of the included studies limits the evidence of this systematic review [869].
Symptomatic obstruction (recurrent flank pain, UTI) requires surgical correction using a pyeloplasty, according to the standardised open technique of Hynes and Anderson [870]. In experienced hands, laparoscopic or retroperitoneoscopic techniques and robot-assisted techniques have the same success rates as standard open procedures. In asymptomatic cases, conservative follow-up is the treatment of choice. A recent interventional study suggested that, in operated infants less than six months, inserting a stent (transanastomotic stent) decreases the complication rates compared to stentless approach [871]. However the results should be taken cautiously since there are successful reported stentless procedures in other age groups.
Indications for surgical intervention comprise impaired split renal function (< 40%), a decrease of split renal function of > 10% in subsequent studies, poor drainage function after the administration of furosemide, increased anteroposterior diameter on US, and grade III and IV dilatation as defined by the Society for Fetal Urology [660].
Well-established benefits of conventional laparoscopy over open surgery are the decreased length of hospital stay, better cosmesis, less post-operative pain and early recovery [872,873]. A recent meta-analysis in children has shown that laparoscopic pyeloplasty (LP) was associated with decreased length of hospital stay and complication rates but prolonged operative time when compared to open pyeloplasty (OP). Additionally, both LP and OP had equal success rates [874]. Laparoscopic pyeloplasty can also be performed for re-do cases with the same advantages of the primary cases [875]. Robotic-assisted laparoscopic pyeloplasty (RALP) has all the same advantages as LP plus better manoeuvrability, improved vision, ease in suturing and increased ergonomics but higher costs [876,877]. A recent study comparing RALP and LP has shown similar postoperative outcomes with exception of decreased operative time for RALP [878]. There does not seem to be any clear benefit of minimal invasive procedures in a very young child but current data is insufficient to defer a cut-off age.
3.13.3.3. Megaureter
The treatment options of secondary megaureters are reviewed in Chapter 3.14.3.
3.13.3.3.1. Non-operative management
If a functional study reveals and confirms adequate ureteral drainage, conservative management is the best option. Initially, low-dose prophylactic antibiotics within the first year of life are recommended for the prevention of UTIs, although there are no existing prospective randomised trials evaluating the benefit of this regimen [879]. With spontaneous remission rates of up to 85% in primary megaureter cases, surgical management is no longer recommended, except for megaureters with recurrent UTIs, deterioration of split renal function and significant obstruction [880].
3.13.3.3.2. Surgical management
In general, surgery is indicated for symptomatic children, if there is a drop in function in conservative follow-up and hydroureteronephrosis is increasing [881]. Data suggest that children with a ureteric diameter of > 10-15 mm are more likely to require intervention [882].
The initial approach to the ureter can be either intravesical, extravesical or combined. Straightening the ureter is necessary without devascularisation. Ureteral tapering should enhance urinary flow into the bladder. The ureter must be tapered to achieve a diameter for an anti-reflux repair. Several tailoring techniques exist, such as ureteral imbrication or excisional tapering [883]. Some institutions perform endoscopic stenting, but there are still no long-term data and no prospective randomised trials to confirm their outcome. A systematic review assessed the success rates of endoscopic management of primary obstructive megaureters [884]. It was reported that endoscopic managements including; stent placement, balloon dilatation and incision can be an alternative treatment in patients > 1 years of age. One third of those patients required further surgical correction. Furthermore, the long-term outcome of endoscopic management is still unknown. Therefore the EAU Paediatric Urology Guidelines Panel can not recommend endoscopic management routinely since the type of intervention and the management outcomes are unclear.
3.13.4. Conclusion
The use of routine perinatal sonography has resulted in increased detection of hydronephrosis caused by UPJ or UVJ obstruction. Meticulous and repeat postnatal evaluation is mandatory to try to identify obstructive cases at risk of renal deterioration and requiring surgical reconstruction. Surgical methods are standardised and have a good clinical outcome.
3.13.5. Summary of evidence and recommendations for the management of UPJ-, UVJ-obstruction
Summary of evidence | LE |
Nowadays, most hydronephrotic kidneys have already been diagnosed prenatally during a maternal US investigation. | 2 |
Ureteropelvic junction obstruction is the leading pathological cause of hydronephrotic kidneys (40%). | 1 |
In children diagnosed with antenatal hydronephrosis, a systematic review could not establish any benefits or harms related to continuous antibiotic prophylaxis. | 1b |
In children diagnosed with antenatal hydronephrosis, non-circumcised infants (LE: 1a), children diagnosed with high-grade hydronephrosis (LE: 2) and hydroureteronephrosis (LE: 1b) were shown to be at higher risk of developing UTI. | 2 |
Recommendations | LE | Strength rating |
Include serial ultrasound (US) and subsequent diuretic renogram and sometimes voiding cystourehrography in postnatal investigations. | 2 | Strong |
Offer continuous antibiotic prophylaxis to the subgroup of children with antenatal hydronephrosis who are at high risk of developing urinary tract infection like uncircumcised infants, children diagnosed with hydroureteronephrosis and high-grade hydronephrosis, respectively. | 2 | Weak |
Decide on surgical intervention based on the time course of the hydronephrosis and the impairment of renal function. | 2 | Weak |
Offer surgical intervention in case of an impaired split renal function due to obstruction or a decrease of split renal function in subsequent studies and increased anteroposterior diameter on the US, and grade IV dilatation as defined by the Society for Fetal Urology. | 2 | Weak |
Offer pyeloplasty when ureteropelvic junction obstruction has been confirmed clinically or with serial imaging studies proving a substantially impaired or decrease in function. | 2 | Weak |
Do not offer surgery as a standard for primary megaureters since the spontaneous remission rates are as high as 85%. | 2 | Strong |
3.14. Vesicoureteric reflux
Lack of robust prospective RCTs limits the strength of the established guidelines for the management of VUR. The scientific literature for reflux disease is still limited and thus the level of evidence is generally low. Most of the studies are retrospective, include different patient groups, and have poor stratification of quality. Also, there is a high risk of presenting misleading results by combining different types of studies when systematically extracting data. Therefore, for reflux disease, it is unfortunately not possible to produce recommendations based on high-quality studies.
These Guidelines aim to provide a practical approach to the treatment of VUR based on risk analysis and selective indications for both diagnostics and intervention. Although the Panel have tried to summarise most of the possible scenarios in one single table, the table itself is still quite busy. The Panel strongly share the view that making simple and practical guidelines would underestimate the complexity of VUR as a sign of a wide range of pathologies [885].
3.14.1. Epidemiology, aetiology and pathophysiology
Vesicoureteric reflux is an anatomical and/or functional disorder with potentially serious consequences, such as renal scarring, hypertension and renal failure. Patients with VUR present with a wide range of severity, and a good proportion of reflux patients do not develop renal scars and probably do not need any intervention [886]. Vesicoureteric reflux is a very common urological anomaly in children, with an incidence of nearly 1%.
Genetic analysis studies revealed monogenic causes for VUR and significant differentiation of innate immunity and epithelial function genes in children with VUR/UTIs compared to controls [887-889]. The main management goal is the preservation of kidney function, by minimising the risk of pyelonephritis. By defining and analysing the risk factors for each patient (i.e. age, sex, reflux grade, LUTD, anatomical abnormalities, and kidney status), it is possible to identify those patients with a potential risk of UTIs and renal scarring. Controversy persists over the optimal management of VUR, particularly the choice of diagnostic procedures, treatment (medical, endoscopic or surgical), and the timing of treatment.
Many children present without symptoms of UTI and, because invasive diagnostic procedures are performed only when clinically indicated, the exact prevalence of VUR is unknown. However, the prevalence of VUR in non-symptomatic children has been estimated at 0.4-1.8% [890]. Among infants prenatally identified with hydronephrosis on US, who were screened for VUR, the prevalence was 16.2% (7-35%) [891]. Siblings of children with VUR had a 27.4% (3-51%) risk of also having VUR, whereas the offspring of parents with VUR had a higher incidence of 35.7% (21.2-61.4%) [891].
However, reflux detected by sibling screening is associated with lower grades [796] and significantly earlier resolution [892]. When VUR is discovered in siblings after UTI, it is usually high-grade and associated with a high incidence of reflux nephropathy, particularly if the sibling is male and the grade of reflux was high in the index patient [893].
The incidence of VUR is much higher among children with UTIs (30-50%, depending on age). Urinary tract infections are more common in girls than boys due to anatomical differences. However, among all children with UTIs, boys are more likely to have VUR than girls (29% vs. 14%). Boys also tend to have higher grades of VUR diagnosed at younger ages, although their VUR is more likely to resolve itself [894-897].
There is a clear co-prevalence between LUTD and VUR [898]. Lower urinary tract dysfunction refers to the presence of LUTS, including urge, urge incontinence, weak stream, hesitancy, frequency and UTIs, which reflect the filling and/or emptying dysfunction and may be accompanied with bowel problems [898]. Some studies have described a prevalence of 40-60% for VUR in children with LUTD [899]. A published Swedish Reflux trial has demonstrated LUTD in 34% of patients, and subdivision into groups characteristic of children revealed that 9% had isolated overactive bladder and 24% had voiding phase dysfunction [900].
The spontaneous resolution of VUR is dependent on age at presentation, sex, grade, laterality, mode of clinical presentation, and anatomy [901]. Faster resolution of VUR is more likely with age less than one year at presentation, lower grade of reflux (grade 1-3), and asymptomatic presentation with prenatal hydronephrosis or sibling reflux. The overall resolution rate is high in congenital high-grade VUR during the first years of life. In several Scandinavian studies, the complete resolution rate for high-grade VUR has been reported at > 25%, which is higher than the resolution rate for VUR detected after infancy [900,902,903].
The presence of renal cortical abnormality, bladder dysfunction, and breakthrough febrile UTIs are negative predictive factors for reflux resolution [904-906].
Dilating VUR increases the risk of developing acute pyelonephritis and renal scarring. Untreated recurrent UTIs may have a negative impact on somatic growth and medical status of the child. Evidence of renal scarring is present in 10-40% of children with symptomatic VUR, resulting from either congenital dysplasia and/or acquired post-infectious damage, which may have a negative impact on somatic growth and general well-being [907-909].
Scar rates vary in different patient groups. Patients with higher grades of VUR present with higher rates of renal scars. In those with prenatal hydronephrosis, renal scarring occurs in 10% of patients [910-915], whereas in patients with LUTD, this may increase up to 30% [673,909,916]. Renal scarring may adversely affect renal growth and function, with bilateral scarring increasing the risk of insufficiency. Reflux nephropathy (RN) may be the most common cause of childhood hypertension. Follow-up studies have shown that 10-20% of children with RN develop hypertension or end-stage renal disease [917].
3.14.2. Diagnostic evaluation
The diagnostic work-up should aim to evaluate the overall health and development of the child, the presence of UTIs, renal status, the presence of VUR, and LUT function. A basic diagnostic work-up comprises a detailed medical history (including family history, and screening for LUTD), physical examination including blood pressure measurement, urinalysis (assessing proteinuria), urine culture, and serum creatinine in patients with bilateral renal parenchymal abnormalities.
The standard imaging tests include renal and bladder US, VCUG and nuclear renal scans. Ultrasound and VCUG could be considered as complementary techniques [918]. The criterion standard in diagnosis of VUR is VCUG, especially at the initial work-up. This test provides precise anatomical detail and allows grading of VUR [919]. In 1985, the International Reflux Study Committee introduced a uniform system for the classification of VUR [920,921] (Table 2). The grading system combines two earlier classifications and is based upon the extent of retrograde filling and dilatation of the ureter, renal pelvis and calyces on VCUG [921].
Radionuclide studies for detection of reflux have lower radiation exposure than VCUG, but the anatomical details depicted are inferior [922]. Recent studies on alternative imaging modalities for detection on VUR have yielded good results with voiding US and magnetic resonance VCUG [923-926]. Contrast enhanced voiding urosonography (ceVUS) with intravesical instillation of different ultrasound contrast agents has been shown to be highly sensitive giving comparable results with conventional VCUG while avoiding exposure to ionising radiation [479,927-929]. However, despite the concerns about ionising radiation and its invasive nature, conventional VCUG still remains the gold standard because it allows better determination of the grade of VUR (in a single or duplicated kidney) and assessment of the bladder and urethral configuration. Intrarenal reflux (IRR) is associated with renal scarring development and it can be diagnosed on the images acquired during the voiding phase of the standard 4-staged VCUG and on ceVUS [930,931].
Table 2: Grading system for VUR on VCUG, according to the International Reflux Study Committee.
Grade I | Reflux does not reach the renal pelvis; varying degrees of ureteral dilatation |
Grade II | Reflux reaches the renal pelvis; no dilatation of the collecting system; normal fornices |
Grade III | Mild or moderate dilatation of the ureter, with or without kinking; moderate dilatation of the collecting system; normal or minimally deformed fornices |
Grade IV | Moderate dilatation of the ureter with or without kinking; moderate dilatation of the collecting system; blunt fornices, but impressions of the papillae still visible |
Grade V | Gross dilatation and kinking of the ureter, marked dilatation of the collecting system; papillary impressions no longer visible; intraparenchymal reflux |
Dimercaptosuccinic acid is the best nuclear agent for visualising the cortical tissue and differential function between both kidneys. Dimercaptosuccinic acid is taken up by proximal renal tubular cells and is a good indicator of renal parenchyma function. In areas of acute inflammation or scarring, DMSA uptake is poor and appears as cold spots. Dimercaptosuccinic acid scans are therefore used to detect and monitor renal scarring. A baseline DMSA scan at the time of diagnosis can be used for comparison with successive scans later during follow-up [933]. Dimercaptosuccinic acid can also be used as a diagnostic tool during suspected episodes of acute pyelonephritis [934]. Children with a normal DMSA scan during acute UTI have a low-risk of renal damage [934,935].
Video-urodynamic studies are only important in patients in whom secondary reflux is suspected, such as those with spina bifida or boys in whom VCUG is suggestive of PUV. In the case of LUTS, diagnosis and follow-up can be limited to non-invasive tests (e.g. voiding charts, US, or uroflowmetry) [898]. Cystoscopy has a limited role in evaluating reflux, except for infravesical obstruction or ureteral anomalies that might influence therapy.
3.14.2.1. Recommendations for diagnosis of VUR
Recommendations | Strength rating |
For diagnosis of vesicoureteric reflux apart from voiding cystourethrogram, contrast enhanced voiding urosonography is another option. | Weak |
3.14.2.2. Infants presenting with prenatally diagnosed hydronephrosis
Ultrasound of the kidney and bladder is the first standard evaluation tool for children with prenatally diagnosed hydronephrosis. It is non-invasive and provides reliable information regarding kidney structure, size, parenchymal thickness and collecting system dilatation [936,937].
Ultrasound should be delayed until the first week after birth because of early oliguria in the neonate. It is essential to evaluate the bladder, as well as the kidneys. The degree of dilatation in the collecting system under US, when the bladder is both full and empty, may provide significant information about the presence of VUR. Bladder wall thickness and configuration may be an indirect sign of LUTD and reflux. The absence of hydronephrosis on postnatal US excludes the presence of significant obstruction; however, it does not exclude VUR.
Monitoring with careful US avoids unnecessary invasive and irradiating examinations. The first two US scans within the first one to two months of life are highly accurate for defining the presence or absence of renal pathology. In infants with two normal, successive scans, VUR is rare, and if present it is likely to be low-grade [910,938]. The degree of hydronephrosis is not a reliable indicator for the presence of VUR, even though cortical abnormalities are more common in high-grade hydronephrosis [891]. The presence of cortical abnormalities on US (defined as cortical thinning and irregularity, as well as increased echogenicity) warrants the use of VCUG for detecting VUR [891]. Dimercaptosuccinic acid provides more reliable and quantitative measurement of the degree of cortical abnormalities, first detected with US.
The use of VCUG is recommended in patients with US findings of bilateral high-grade hydronephrosis, duplex kidneys with hydronephrosis, ureterocele, ureteric dilatation, and abnormal bladders, because the likelihood of VUR is much higher. In all other conditions, the use of VCUG to detect reflux is optional [891,912,939-941].
When infants who are diagnosed with prenatal hydronephrosis become symptomatic with UTIs, further evaluation with VCUG should be considered [940]. Patients with severe hydronephrosis and those whose hydronephrosis is sustained or progressive, need further evaluation to exclude obstruction.
3.14.2.3. Siblings and offspring of reflux patients
The screening of asymptomatic siblings and offspring is controversial. Some authors think that early identification of children with VUR may prevent episodes of UTI and therefore renal scarring, whereas others think that screening asymptomatic individuals is likely to result in significant over-treatment of clinically insignificant VUR. In screened populations the prevalence of VUR is 27.4% in siblings and 35.7% in offspring [932]. The overall estimate for renal cortical abnormalities is 19.3% (11-54%), with 27.8% having renal damage in cohorts of symptomatic and asymptomatic children combined. In asymptomatic siblings only, the rate of renal damage is 14.4% (0-100%). Although early screening and therefore early diagnosis and treatment appears to be more effective than late screening in preventing further renal damage [891,893,942,943], screening in all siblings and offspring cannot be recommended based on the available evidence. The lack of RCTs for screened patients to assess clinical health outcomes makes evidence-based guideline recommendations difficult.
3.14.2.4. Recommendations for paediatric screening of VUR
Recommendations | Strength rating |
Inform parents of children with vesicoureteric reflux (VUR) that siblings and offspring have a high prevalence of VUR. | Strong |
3.14.2.5. Children with febrile urinary tract infections
A routine recommendation of VCUG at zero to two years of age, after the first proven febrile UTI is the safest approach as the evidence for the criteria to selecting patients for reflux detection is weak. Upon diagnosing a child with the first febrile UTI, the risk factors: age (> 6 months), presence of sepsis, WBC count (> 15 000/mm), and abnormal renal US results, can be used for the generation of a predictive score for VUR presence [944]. (See Section 3.9 on urinary tract infections in children).
Children with febrile infections and abnormal renal US findings may have higher risk of developing renal scars and they should all be evaluated for reflux [482]. If reflux is diagnosed, further evaluation has traditionally consisted of a DMSA scan.
An alternative “top-down” approach is also an option, as suggested by several studies in the literature. This approach carries out an initial DMSA scan close to the time of a febrile UTI, to determine the presence of pyelonephritis, which is then followed by VCUG if the DMSA scan reveals kidney involvement. A normal DMSA scan with no subsequent VCUG will fail to identify VUR in 5-27% of cases, with the missed VUR presumably being less significant. In contrast, a normal DMSA scan with no VCUG avoids unnecessary VCUG in > 50% of those screened [472,945-947].
3.14.2.6. Children with lower urinary tract symptoms and vesicoureteric reflux
Detection of LUTD is essential in treating children with VUR. It is suggested that reflux with LUTD resolves faster after LUTD correction, and that patients with LUTD are at higher risk for developing UTI and renal scarring [897,948]. The co-existence of both conditions should be explored in any patient who has VUR. If there are symptoms suggestive of LUTD (e.g. urgency, wetting, constipation or holding manoeuvres), an extensive history and examination, including voiding charts, uroflowmetry and residual urine determination, will reliably diagnose underlying LUTD.
Among toilet-trained children, those with both LUTD and VUR are at higher risk of developing recurrent UTIs than children with isolated VUR [509]. Bladder and bowel dysfunction is common in toilet-trained children presenting with UTI with or without primary VUR. A subgroup meta-analysis also shows that functional constipation is common in these children, with almost every third child affected by it. It was also found that the presence of both BBD and VUR doubles the risk of recurrence of UTI; hence, all children presenting with UTI should be carefully evaluated for presence of BBD and managed accordingly [949].
In LUTD, VUR is often low-grade and US findings are normal, and there is no indication for performing VCUG in all children with LUTD, but the presence of febrile infections should be meticulously investigated. The co-existence of LUTD and VUR means it would be better to do a test covering both conditions, such as a VUDS. Any patient with LUTD and a history of febrile UTI should be investigated with a VUDS, if available. Furthermore, any child who fails standard therapy for LUTD should undergo urodynamic investigation. At this stage, combining a urodynamic study with VCUG is highly recommended.
3.14.3. Disease management
There are two main treatment approaches: conservative (non-surgical and surgical).
3.14.3.1. Non-surgical therapy
The objective of conservative therapy is prevention of febrile UTI. It is based on the understanding that:
- Vesicoureteric reflux can resolve spontaneously, mostly in young patients with low-grade reflux. Renal scarring is also a significant risk factor for breakthrough UTI and could be used to determine those at risk of symptomatic VUR persistence [950];
- Resolution is nearly 80% in VUR grades I and II and 30-50% in VUR grades III-V within four to five years of follow-up;
- Spontaneous resolution is low for bilateral high-grade reflux [951];
- Vesicoureteric reflux is very unlikely to damage the kidney postnatally when patients are free of infection and have normal LUT function;
- There is no evidence that small scars even bilateral can cause hypertension, renal insufficiency or problems during pregnancy. Indeed, these are possible only in cases of severe bilateral renal damage;
- The conservative approach includes watchful waiting, intermittent or CAP, and bladder and bowel rehabilitation in those with LUTD [673,948,952-954];
- Circumcision during early infancy may be considered as part of the conservative approach as it is effective in reducing the risk of infection in normal children [955].
3.14.3.1.1. Follow-up
Regular follow-up with imaging studies (e.g. VCUG, nuclear cystography, or DMSA scan) is part of the conservative management to monitor spontaneous resolution and kidney status. Vesicoureteral reflux increases the risk of febrile UTI and renal scarring especially when in combination with LUTD. Constipation in VUR patients with UTI is common and the prevelence can reach 27%. Assessment and management of all toilet trained children presenting with UTI should be a part of conservative follow-up [949]. During the conservative management of high-grade infant reflux, spontaneous downgrading and resolution of VUR is more likely. However this also depends on gender, breakthrough UTI, renal damage type and bladder dysfunction. Practical scoring systems for making decisions on further treatment, surveillance, prophylaxis or surgical intervention exist [956]. Conservative management should be dismissed in all cases of febrile breakthrough infections, despite prophylaxis, and intervention should be considered.
3.14.3.1.2. Continuous antibiotic prophylaxis
Many prospective studies have evaluated the role of CAP in the prevention of recurrent UTI and renal scarring.
It is clear that antibiotic prophylaxis may not be needed in every reflux patient [957-959]. Trials show the benefit of CAP is none or minimal in low-grade reflux. Continuous antibiotic prophylaxis is useful in patients with grade III and IV reflux in preventing recurrent infections but its use in preventing further renal damage is not proven. For VUR children receiving CAP, younger age at the initial diagnosis of UTI (< 12 months), bilateral VUR, and BBD are independent risk factors for the occurrence of break through UTIs [960]. Toilet-trained children and children with LUTD derive better benefit from CAP [959,961-965]. The RIVUR trial was the largest, randomised, placebo-controlled, double blind, multi-centre study, involving 607 children aged 2-72 months with grade I-IV VUR. The RIVUR study showed that prophylaxis reduced the risk of recurrent UTI by 50% but not renal scarring and its consequences (hypertension and renal failure), at the cost of increased antimicrobial resistance. The benefit of prophylaxis was insignificant in patients with grade III or IV VUR and in the absence of LUTD [966-969]. Additional review of the RIVUR data based on a risk classification system defines a high-risk group (uncircumcised males; presence of BBD and high grade reflux) who would benefit from a antibiotic prophylaxis significantly. In the context of management with CAP in VUR patients, this should be viewed as a spectrum and a shift from ‘absolute’ CAP in dilated VUR towards a ‘selective’ risk-based approach and should be supported [970]. It may be difficult and risky to select patients who do not need CAP. A safe approach would be to use CAP in most cases. Decision-making may be influenced by the presence of risk factors for UTI, such as young age, high-grade VUR, status of toilet-training/LUTS, female sex, and circumcision status. Although the literature does not provide any reliable information about the duration of CAP in reflux patients, a practical approach would be to use CAP until after children have been toilet-trained and ensuring that there is no LUTD.
The literature generaly consists of prescribing daily antibiotics at one quarter to one half the regular therapeutic dose. Trimethoprim-sulfamethoxazole, amoxicilin and nitrofurantoin are the most commonly used CAP agents. A child with a UTI and significant VUR can still be recommended to be treated conservatively at first, with surgical care reserved for incompliance for CAP, breakthrough UTIs under CAP and significant VUR that persists of long-term follow-up [960,971].
Determination of optimal timing to discontinue CAP is controversial however patients administered CAP for less than a year after the last febrile UTI and those with bilateral VUR are likely to have more frequent recurrence. Administration of CAP more than one year after the last febrile UTI can potentially be beneficial to avoid recurrent UTIs [972]. Active surveillance of UTI is needed after CAP is discontinued. The follow-up scheme and the decision to perform an anti-reflux procedure or discontinuation of CAP should be tailored for each VUR case together with the patient and caregivers. It is strongly advised that the advantages and disadvantages should be discussed in detail and easy/early access to healthcare during febrile UTIs should be taken into consideration.
One of the biggest concerns of CAP for patients, caregivers and physicians is the long-term effects of CAP. As a secondary outcome of the RIVUR study, TMP-SMZ prophylaxis for two years did not reveal any adverse effect on complete blood count (CBC), serum electrolytes and creatinine and such routine laboratory tests in otherwise healthy children is not mandatory [973]. Impact of long-term CAP on gut microbiota in children with VUR is contraversial and requires more research [974,975].
Continuous antibiotic prophylaxis, for prevention of UTIs in symptomatic VUR, which diagnosed during the work-up of ANH, is recommended in the first year of life. However, the current literature remains unclear whether infants diagnosed with asypmtomatic VUR during the ANH work-up will also benefit from
CAP [976].
Recommendations | Strength rating |
Initially treat all symptomatic patients diagnosed within the first year of life with continuous antibiotic prophylaxis, regardless of the grade of reflux or presence of renal scars. | Weak |
Offer immediate, parenteral antibiotic treatment for febrile breakthrough infections. | Strong |
Initially manage all children presenting at age one to five years conservatively. | Strong |
Offer close surveillance without antibiotic prophylaxis to children presenting with lower grades of reflux and without symptoms. | Strong |
Ensure that a detailed investigation for the presence of lower urinary tract dysfunction (LUTD) is done in all and especially in children after toilet-training. If LUTD is found, the initial treatment should always be for LUTD. | Strong |
3.14.3.2. Surgical treatment
Surgical treatment can be carried out by endoscopic injection of bulking agents or ureteral re-implantation.
3.14.3.2.1. Subureteric injection of bulking materials
With the availability of biodegradable substances, endoscopic subureteric injection of bulking agents has become an alternative to long-term antibiotic prophylaxis and open surgical intervention in the treatment of VUR in children. Using cystoscopy, a bulking material is injected beneath the intramural part of the ureter in a submucosal location. The injected bulking agent elevates the ureteral orifice and supports the distal ureter, lengthens the submuscosal tunnel so that coaptation is increased. This results in narrowing of the lumen, which prevents reflux of urine into the ureter, while still allowing its antegrade flow. Reflux timing during VCUG can be used to predict the success rate of endoscopic treatment, since reflux occuring only during the voiding phase has a higher success than filling phase VUR [977].
Several bulking agents have been used over the past two decades, including polytetrafluoroethylene (PTFE or Teflon™), collagen, autologous fat, polydimethylsiloxane, silicone, chondrocytes, a solution of dextranomer/hyaluronic acid (D/HA) (Deflux™, Dexell®) and more recently polyacrylatepolyalcohol copolymer hydrogel (PPC) (Vantris®) [978,979].
Although the best results have been obtained with PTFE [980], due to concerns about particle migration, PTFE has not been approved for use in children [981]. Although they are all biocompatible, other compounds such as collagen and chondrocytes have failed to provide a good outcome. Deflux™ was approved by the USA FDA in 2001 for the treatment of VUR in children. Injection can be performed under the ureteric orifice to create a volcanic appearance or by using a hydrodistension technique to the ureteric orifice followed by injection to the intramural ureter.
In a meta-analysis [982] of 5,527 patients and 8,101 renal units, the reflux resolution rate (by ureter) following one treatment for grades I and II reflux was 78.5%, 72% for grade III, 63% for grade IV, and 51% for grade V. If the first injection was unsuccessful, the second treatment had a success rate of 68% and the third treatment 34%. The aggregate success rate with one or more injections was 85%. The success rate was significantly lower for duplicated (50%) vs. single (73%) systems, and neuropathic (62%) vs. normal (74%) bladders. The required injection volume of PPC and D/HA to achieve the same success rate can differ between agents and is generally less for PPC [983,984].
Ureteral diameter ratio is a relatively recent objective measurement and appears to be a new predictive tool for clinical outcome and success after endoscopic injection of VUR [985].
Obstruction at UVJ (UVJO) may happen in the long term follow-up after endoscopic correction of reflux. Patients with high-grade reflux and dilated ureters are at risk of late obstruction. Although in the short term (3-6 months) follow-up success rates and UVJ obstruction seems to be comparable in the long run, it is significantly more common when polyacrylate-polyalcohol copolymer is used as bulking substance [986-989]. The ureteral reimplantation following a failed endoscopic surgery is more challenging after PPC and distal ureter can not be preserved and requires excision due to fibrosis [984]. Although ureteral fibrosis or inflamatory changes following Vantris injection causing UVJO has been shown to be similar to other injection materials, still PPC demonstrates a higher obstruction rate [990].
Clinical validation of the effectiveness of anti-reflux endoscopy is currently hampered by the lack of methodologically appropriate studies. In the most recent prospective, randomised trials comparing three treatment arms: i) endoscopic injection; ii) antibiotic prophylaxis; iii) surveillance without antibiotic prophylaxis in 203 children aged one to two years with grade III/IV reflux, endoscopic treatment gave the highest resolution rate of 71% compared to 39% and 47% for treatment arms ii and iii, respectively, after two years’ follow-up. The recurrence rate at two years after endoscopic treatment was 20%. The occurrence of febrile UTIs and scar formation was highest in the surveillance group at 57% and 11%, respectively. New scar formation rate was higher with endoscopic injection (7%) compared with antibiotic prophylaxis (0%) [991]. Longer follow-up studies are needed to validate these findings.
High-grade VUR in infants can be treated with injection therapy and the resolution rate is higher than that of prophylaxis. However, this can not be recommended for all high-grade infants with VUR since not all are symptomatic and also resolution or downgrading can be achieved with favourable conditions such as unilaterality, grade IV and low residual urine [992,993].
3.14.3.2.2. Open surgical techniques
Various intra- and extravesical techniques have been described for the surgical correction of reflux. Although different methods have specific advantages and complications, they all share the basic principle of lengthening the intramural part of the ureter by submucosal embedding of the ureter. All techniques have been shown to be safe with a low rate of complications and excellent success rates (92-98%) [994].
The most popular and reliable open procedure is cross trigonal re-implantation described by Cohen [988]. The main concern with this procedure is the difficulty of accessing the ureters endoscopically, if needed, when the child is older. Alternatives are suprahiatal reimplantation (Politano-Leadbetter technique) and infrahiatal re-implantation (Glenn-Anderson technique). If an extravesical procedure (Lich-Gregoir) is planned, cystoscopy should be performed pre-operatively to assess the bladder mucosa and the position and configuration of the ureteric orifices. In bilateral reflux, an intravesical anti-reflux procedure may be considered, because simultaneous bilateral extravesical reflux repair carries an increased risk of temporary post-operative urine retention [995]. Overall, all surgical procedures offer very high and similar success rates for correcting VUR.
3.14.3.2.3. Laparoscopy and robot-assisted
There have been a considerable number of case series of transperitoneal, extravesical and pneumovesicoscopic intravesical ureteral re-implantation, which have shown the feasibility of the techniques. A recent systemic review and meta-analysis comparing laparoscopic extravesical (LEVUR) vs. transvesicoscopic ureteral reimplantation (TVUR), revealed both to be good alternatives in terms of success and complication rates. Laparoscopic extravesical ureteral reimplantation is generally biasly preferred for unilateral low grade cases and therefore appears to have a higher success and shorter hospital stay [989].
Various anti-reflux surgeries have been performed with the robot and the extravesical approach is the most commonly used. Although initial reports give comparable outcomes to their open surgical counterparts in terms of successful resolution of reflux, meta-analysis of results of robotic-assisted laparoscopic ureteral reimplantation (RALUR) are within a wide range of variation and on average they are poor compared to open surgery. Operative times, costs and post-operative complications leading to secondary interventions are higher with RALUR but post-operative pain and hospital stay is less compared to open surgery [996-999].
In addition, laparoscopic- or robotic-assisted approaches are more invasive than endoscopic correction and their advantages over open surgery are still debated. Therefore, at present, a laparoscopic approach cannot be recommended as a routine procedure. It can be offered as an alternative to the caregivers in centres where there is established experience [955,1000-1008]. Older children with complex anatomy and/or following a failed injection or open reimplant, can specifically benefit from RALUR since the robotic approach can facilitate the exposure. Robotic-assisted laparoscopic ureteral reimplantation can be performed uni or bilateral, although caution is advised in bilateral cases due to the risk of transient retention [997].
De novo hydronephrosis up to 30% can occur after extravesical RALUR and behave similarly to open ureteral reimplantation which is self resolving in the overwhelming majority of cases [1009].
3.14.4. Summary of evidence and recommendations for the management of vesicoureteric reflux in childhood
Summary of evidence | LE |
There is no evidence that correction of persistent low-grade reflux (grades I-III) without symptoms and normal kidneys offers a significant benefit. | 3 |
The traditional approach of initial medical treatment after diagnosis and shifting to interventional treatment in case of breakthrough infections and new scar formation needs to be challenged, because the treatment should be tailored to different risk groups. | 4 |
Surgical correction should be considered in patients with persistent high-grade reflux (grades IV/V). There is no consensus about the timing and type of surgical correction. The outcome of reimplantation is better than endoscopic correction for higher grades of reflux, whereas satisfactory results can be achieved by endoscopic injection for lower grades. | 3 |
The choice of management depends on the presence of renal scars, clinical course, grade of reflux, ipsilateral renal function, bilaterality, bladder function, associated anomalies of the urinary tract, age, compliance, and parental preference. Febrile UTI, high-grade reflux, bilaterality, and cortical abnormalities are considered to be risk factors for possible renal damage. The presence of LUTD is an additional risk factor for new scars. | 4 |
Recommendations | Strength rating |
Offer reimplantation or endoscopic correction to patients with frequent breakthrough infections. | Weak |
Offer reimplantation to patients with persistent high-grade reflux and endoscopic correction for lower grades of reflux. | Strong |
Offer surgical repair to children above the age of one presenting with high-grade reflux and abnormal renal parenchyma. | Weak |
Offer surgical correction, if parents prefer definitive therapy to conservative management. | Strong |
Select the most appropriate management option based on: the presence of renal scars; clinical course; the grade of reflux; ipsilateral renal function; bilaterality; bladder function; associated anomalies of the urinary tract; age and gender; compliance; parental preference. Refer to Table 3 for risk factors and follow-up. | Weak |
In high-risk patients who already have renal impairment, a more aggressive, multi-disciplinary approach is needed. | Strong |
Table 3: Management and follow-up according to different risk groups
Risk Groups | Presentation | Initial treatment | Comment | Follow-up |
High | Symptomatic male or female patients after toilet-training with high-grade reflux (grades IV-V), abnormal kidneys and LUTD | Initial treatment is always for LUTD with CAP; intervention may be considered in cases of BT infections or persistent reflux | Greater possibility of earlier intervention | More aggressive follow-up for UTI and LUTD; full re-evaluation after |
High | Symptomatic male or female patients after toilet-training with high-grade reflux (grade IV-V), abnormal kidneys and no LUTD | Intervention should be considered | Reimplantation has better results than endoscopic surgery | Post-operative VCUG on indication only; follow-up of kidney status until after puberty |
Moderate | Symptomatic male or female patients before toilet-training, with high-grade reflux and abnormal kidneys | CAP is the initial treatment. Intervention may be considered in cases of BT infections or persistent reflux | Spontaneous resolution is higher in males | Follow-up for UTI/ hydronephrosis; full re-evaluation after 12-24 months |
Moderate | Asymptomatic patients (PNH or sibling) with high-grade reflux and abnormal kidneys | CAP is the initial treatment. Intervention may be considered in cases of BT, infections or persistent reflux | Follow-up for UTI/ hydronephrosis; full re-evaluation after 12-24 months | |
Moderate | Symptomatic male or female patients after toilet-training, with high-grade reflux and normal kidneys with LUTD | Initial treatment is always for LUTD with CAP. Intervention may be considered in cases of BT infections or persistent reflux | In case of persistent LUTD, despite urotherapy, intervention should be considered. The choice of intervention is controversial | Follow-up for UTI and LUTD, kidney status; full re-evaluation after successful urotherapy |
Moderate | Symptomatic male or female patients after toilet-training with low-grade reflux, abnormal kidneys with or without LUTD | Choice of treatment is controversial. Endoscopic treatment may be an option. LUTD treatment should be given if needed | Follow-up for UTI, LUTD, and kidney status until after puberty | |
Moderate | All symptomatic patients with normal kidneys, with low-grade reflux, with LUTD | Initial treatment is always for LUTD with or without CAP | Follow-up for UTI and LUTD | |
Low | All symptomatic patients with normal kidneys, with low-grade reflux, with no LUTD | No treatment or CAP | If no treatment is given, parents should be informed about risk of infection | Follow-up for UTI |
Low | All asymptomatic patients with normal kidneys with low-grade reflux | No treatment or CAP in infants | If no treatment is given, parents should be informed about risk of infection | Follow-up for UTI |
BT = breakthrough; CAP = continuous antibiotic prophylaxis; LUTD = lower urinary tract dysfunction; PNH = prenatal diagnosed hydronephrosis; UTI = urinary tract infection; VCUG = voiding cystourethrography.
3.15. Urinary stone disease
3.15.1. Epidemiology, aetiology and pathophysiology
Paediatric stone disease is an important clinical problem in paediatric urology practice. Due to its recurrent nature, every effort should be made to discover the underlying metabolic abnormality so that it can be treated appropriately. The main goal is to maintain a stone-free state with close follow-up, although it may not be possible in some circumstances (e.g. oxalosis or nephrocalcinosis).
Bladder stones are still common in under-developed areas of the world and are usually ammonium acid urate and uric acid stones, strongly implicating dietary factors [1010,1011]. Hypocitraturia is the most common metabolic abnormality, followed by hypercalciuria [1012]. Patients with augmented bladder constitute another important group with a risk of up to 15% [1013].
The incidence and characteristics of stones show a wide geographical variation in children. Although urinary stone disease is generally considered to be a relatively rare disease, it is quite common in some parts of the world. Paediatric stone disease is endemic in Turkey, Pakistan and in some South Asian, African and South American countries. However, epidemiological studies have shown that the incidence of paediatric stone disease is also increasing in the Western world [1014-1016]; especially in girls, Caucasian ethnicity, African Americans and older children [1017]. More than 70% of stones in children contain calcium oxalate, while infectious stones are found more frequently in younger children [1018]. The risk for stone recurrence among childhood stone formers has been reported to be 35-50%. No sex differences could be found regarding the stone recurrence risk [1019,1020].
3.15.2. Classification systems
Urinary stone formation is the result of a complex process involving genetic, dietary, metabolic, anatomical factors and presence of infection.
3.15.2.1. Calcium stones
Calcium stones are usually made from calcium oxalate or calcium phosphate. Super-saturation of calcium (hypercalciuria) and oxalate (hyperoxaluria) or decreased concentration of inhibitors, such as citrate (hypocitraturia) or magnesium (hypomagnesemia) play a major role in the formation of calcium oxalate stones. Higher super-saturations of calcium oxalate were shown to be associated with multiple stone disease [1021].
Hypercalciuria: This is defined by a 24-hour urinary calcium excretion of more than 4 mg/kg/day (0.1 mmol/
kg/day) in a child weighing < 60 kg. In infants younger than three months, 5 mg/kg/day (0.125 mmol/kg/day) is considered to be the upper limit for normal calcium excretion [1022].
Hypercalciuria can be classified as either idiopathic or secondary. Idiopathic hypercalciuria is diagnosed when clinical, laboratory, and radiographic investigations fail to delineate an underlying cause leading to hypercalcaemia. Urinary calcium may increase in patients with high sodium chloride intake.
Secondary hypercalciuria occurs when a known process produces excessive urinary calcium. In secondary hypercalcaemic hypercalciuria, a high serum calcium level may be due to increased bone resorption (hyperparathyroidism, hyperthyroidism, immobilisation, acidosis, metastatic disease) or gastrointestinal hyperabsorption (hypervitaminosis D) [1023].
A good screening test for hypercalciuria compares the ratio of urinary calcium to creatinine. The normal calcium-to-creatinine ratio in children is less than 0.2. If the calculated ratio is higher than 0.2, repeat-testing is indicated. Neonates and infants have a higher calcium excretion and lower creatinine excretion than older children [1022,1023]. If the follow-up ratios are normal, then no additional testing for hypercalciuria is needed.
However, if the ratio remains elevated, a timed 24-hour urine collection should be obtained and the calcium excretion calculated. The 24-hour calcium excretion test is the standard criterion for the diagnosis of hypercalciuria. If calcium excretion is higher than 4 mg/kg/day (0.1 mmol/kg/day), the diagnosis of hypercalciuria is confirmed and further evaluation is warranted: levels of serum bicarbonate, creatinine, alkaline phosphatase, calcium, phosphorus, magnesium, pH, and parathyroid hormone. Freshly voided urine should be measured for pH [1022-1024]. In addition to calcium, the 24-hour urine analysis should also include phosphorus, sodium, magnesium, uric acid, citrate and oxalate.
Initial management is always to increase fluid intake and urinary flow. Dietary modification is a mandatory part of effective therapy. The child should be referred to a dietician to accurately assess the daily intake of calcium, animal protein, and sodium. Dietary sodium restriction is recommended as well as maintenance of calcium intake consistent with the daily needs of the child [1025]. A brief trial of a low calcium diet can be carried out to determine if exogenous calcium intake and/or calcium hyperabsorption is contributing to high urinary calcium. Any recommendation to restrict calcium intake below the daily needs of the child should be avoided. Moreover, low calcium intake is a risk factor for stone formation [1026] (LE: 3).
Hydrochlorothiazide and other thiazide-type diuretics may be used to treat idiopathic hypercalciuria, especially with calcium renal leak, at a starting dosage of 0.5-1 mg/kg/day [1027-1030] (LE: 3). In long-term use of thiazide-type diuretics, a decrease in hypocalciuric effect may be seen after the third month and may cause hypokalemia, hypocitraturia, hyperuricaemia and hypomagnesaemia. Therefore, control of blood and serum values should be performed in regular intervals. Citrate therapy is also useful if citrate levels are low or if hypercalciuria persists, despite other therapies [1027,1031] (LE: 4).
Hyperoxaluria: Only 10-15% of oxalate is dietary. The average child excretes less than 50 mg (0.57 mmol/
1.73 m2/day) [1032-1034], while infants excrete four times as much. Hyperoxaluria may result from increased dietary intake, enteric hyperabsorption (as in short bowel syndrome) or an inborn error of metabolism.
In rare primary hyperoxaluria, one of the two liver enzymes that play a role in the metabolism of oxalate may be deficient. With increased deposition of calcium oxalate in the kidneys, renal failure may ensue, resulting in deposition of calcium oxalate in other tissues (oxalosis). The diagnosis is made upon laboratory findings of severe hyperoxaluria and clinical symptoms. The definitive diagnosis requires a liver biopsy to assay the enzyme activity. Patients with primary hyperoxaluria exhibit a substantial clinical burden such as renal stones, UTIs and pain, requiring frequent healthcare resource use [1035].
Other forms of hyperoxaluria, as mentioned earlier, may be due to hyperabsorption of oxalate in inflammatory bowel syndrome, pancreatitis and short bowel syndrome. Yet, the majority of children have ‘mild’ (idiopathic) hyperoxaluria, with only mildly elevated urine oxalate levels in these cases. The treatment of hyperoxaluria consists of the promotion of high urine flow, restriction of dietary oxalate and regular calcium intake. Pyridoxine may be useful in reducing urine levels, especially in primary hyperoxaluria. Citrate administration increases inhibitory urine activity [1027,1036] (LE: 4).
Hypocitraturia: Citrate is a urinary stone inhibitor. It acts by binding to calcium and by directly inhibiting the growth and aggregation of calcium oxalate as well as calcium phosphate crystals. Thus low urine citrate may be a significant cause of calcium stone disease. In adults, hypocitraturia is the excretion of urinary citrate of less than 320 mg/day (1.5 mmol/day); this value must be adjusted for children depending on body size [1037-1039].
Hypocitraturia usually occurs in the absence of any concurrent symptoms or any known metabolic derangements. It may also occur in association with any metabolic acidosis, distal tubular acidosis or diarrhoeal syndromes.
Environmental factors that lower urinary citrate include a high protein intake and excessive salt intake. Many reports emphasise the significance of hypocitraturia in paediatric calcium stone disease. The presence of hypocitraturia ranges from 30% to 60% in children with calcium stone disease [1038,1040]. The urine calcium-to-citrate ratios were higher in recurrent calcium stone forming children than solitary formers [1037,1041].
The restoration of normal citrate levels is advocated to reduce stone formation, although there are few relevant studies in children. Hypocitraturia is treated by potassium citrate at a starting dose of 1 mEq/kg, given in two divided doses [1028] (LE: 3). The side effects of potassium citrate are very rare and most of the time they include non-specific gastrointestinal complaints. Potassium citrate should be used with caution in hyperkalemic and chronic renal failure conditions.
3.15.2.2. Uric acid stones
Uric acid stones are responsible for urinary calculi in 4-8% of children. Uric acid is the end product of purine metabolism. Hyperuricosuria is the main cause of uric acid stone formation in children. A daily output of uric acid of more than 10 mg/kg/day (0.6 mmol/kg/day) is considered to be hyperuricosuria [1027]. The formation of uric acid stones is mainly dependent on the presence of an acidic urinary composition. Uric acid dissociation and solubility is strongly reduced at a pH of < 5.8. As the pH becomes more alkaline, uric acid crystals become more soluble and the risk of uric acid stone formation is reduced.
In the familial or idiopathic form of hyperuricosuria, children usually have normal serum uric acid levels. In other children, it can be caused by uric acid overproduction secondary to inborn errors of metabolism, myeloproliferative disorders or other causes of cell breakdown. Hyperuricosuria is also caused by high purine and protein intake. Although hyperuricosuria is a risk factor for calcium oxalate stone formation in adults, this does not appear to be a significant risk factor in children. Uric acid stones are non-opaque stones. Plain x-rays are insufficient to show uric acid stones, and renal sonography and spiral CT are used for diagnosis.
Alkalinisation of urine is the mainstay of therapy and prevention for uric acid stones. Citrate preparations are useful as alkalinising agents. Maintaining a urine pH of 6 to 6.5 is sufficient to prevent uric acid stones [1027]. In patients who failed conservative measures with sustaining hyperuricosuria and hyperuricemia, stone recurrences or myeloproliferative diseases, allopurinol (10 mg/kg) may be used. This medication may cause several drug reactions (rash, diarrhoea, eosinophilia) and should be cautiously used in chronic renal failure patients.
3.15.2.3. Cystine stones
Cystinuria is the cause of cystine stone formation and accounts for 2-6% of all urinary stones in children. Cystinuria is an incompletely recessive autosomal disorder characterised by failure of renal tubules to reabsorb four basic amino acids: cystine, ornithine, lysine and arginine.
Of these four amino acids, only cystine has poor solubility in urine, so that only cystine stones may form in the case of excessive excretion in urine. Cystine solubility is pH-dependent, with cysteine precipitation beginning at pH levels < 7.0. Other metabolic conditions, such as hypercalciuria, hypocitraturia and hyperuricosuria, may accompany cystinuria, so leading to the formation of mixed-composition stones. Cystine stones are faintly radiopaque and may be difficult to visualise on regular radiograph studies. They are also hard in texture and more difficult to disintegrate by extracorporeal shockwave lithotripsy (SWL). Cystinuric patients present with larger stones at the time of diagnosis, higher new stone formation rates, and are at higher risk of surgery [1042].
The medical treatment for cystine stones aims to reduce cystine saturation in urine and increase its solubility. The initial treatment consists of maintaining a high urine flow and the use of alkalinising agents, such as potassium citrate to maintain urine pH at above 7.0 (better above 7.5). If this treatment fails, the use of α-mercaptopropionyl glycine or D-penicillamin may increase cystine solubility and reduce cystine levels in urine and prevent stone formation. Side effects of these drugs are mostly mild and include gastrointestinal complaints (alterations in taste and odour), fever and rash, however they can be associated with severe side effects, such as bone marrow depression, nephrotic syndrome and epidermolysis [1043].
3.15.2.4. Infection stones (struvite stones)
Infection-related stones constitute nearly 5% of urinary stones in children, though incidence increases over 10% in younger ages [1044] and in non-endemic regions [1018,1045]. Bacteria capable of producing urease enzyme (Proteus, Klebsiella, Pseudomonas) are responsible for the formation of such stones.
Urease converts urea into ammonia and bicarbonate, alkalinising the urine and further converting bicarbonate into carbonate. In the alkaline environment, triple phosphates form, eventually resulting in a supersaturated environment of magnesium ammonium phosphate and carbonate apatite, which in turn leads to stone formation.
In addition to bacterial elimination, stone elimination is essential for treatment, as stones will harbour infection and antibiotic treatment will not be effective. Consideration should be given to investigating any congenital problem that causes stasis and infection. Genitourinary tract anomalies predispose to formation of such stones.
3.15.3. Diagnostic evaluation
Presentation tends to be age-dependent, with symptoms such as flank pain and haematuria being more common in older children. Non-specific symptoms (e.g. irritability, vomiting) are common in very young children. Haematuria, usually visible, occurring with or without pain, is less common in children. However, non-visible haematuria may be the sole indicator and is more common in children. In some cases, urinary infection may be the only finding leading to radiological imaging in which a stone is identified [1046,1047].
3.15.3.1. Imaging
Generally, US should be used as a first approach. Renal US is very effective for identifying stones in the kidney. Many radiopaque stones can be identified with a simple abdominal flat-plate examination. The most sensitive test for identifying stones in the urinary system (especially for ureteric stones) is non-contrast helical CT scanning. It is safe and rapid, with 97% sensitivity and 96% specificity [1048-1050]. Despite its high diagnostic accuracy, because of the potential radiation hazards, its use should be reserved for cases with non-informative US and/or plain abdominal radiograph. Low dose protocols have also been developed with the goal of reducing radiation dose with adequate image quality [1051]. Intravenous pyelography is rarely used in children, but may be needed to delineate the caliceal anatomy prior to percutaneous or open surgery.
3.15.3.2. Metabolic evaluation
Due to the high incidence of predisposing factors for urolithiasis in children and high stone recurrence rates, every child with a urinary stone should be given a complete metabolic evaluation [1010,1043,1052-1054]. A limited urinary metabolic evaluation (24-h calcium, 24h-citrate, and 24h-oxalate and low urinary volume) is able to detect the vast majority of clinically significant metabolic abnormalities [1055]. However, most of the time collections are inadequate and have to be repeated [1056].
Metabolic evaluation includes:
- family and patient history of metabolic problems and dietary habits;
- analysis of stone composition (following stone analysis, metabolic evaluation can be modified according to the specific stone type);
- electrolytes, blood/urea/nitrogen (BUN), creatinine, calcium, phosphorus, alkaline phosphatase, uric acid, total protein, carbonate, albumin, and parathyroid hormone (if there is hypercalcaemia);
- spot urinalysis and urine culture, including ratio of calcium to creatinine;
- urine tests, including a 24-hour urine collection for calcium, phosphorus, magnesium, oxalate, uric acid citrate, protein, and creatinine clearance;
- 24-hour cystine analysis if cystinuria is suspected (positive sodium nitroprusside test, cystine stone, cystine hexagonal crystals in urine).
Figure 10 provides an algorithm of how to perform metabolic investigations in urinary stone disease in children
and how to plan medical treatment accordingly.
Figure 10: Algorithm for metabolic investigations in urinary stone disease in children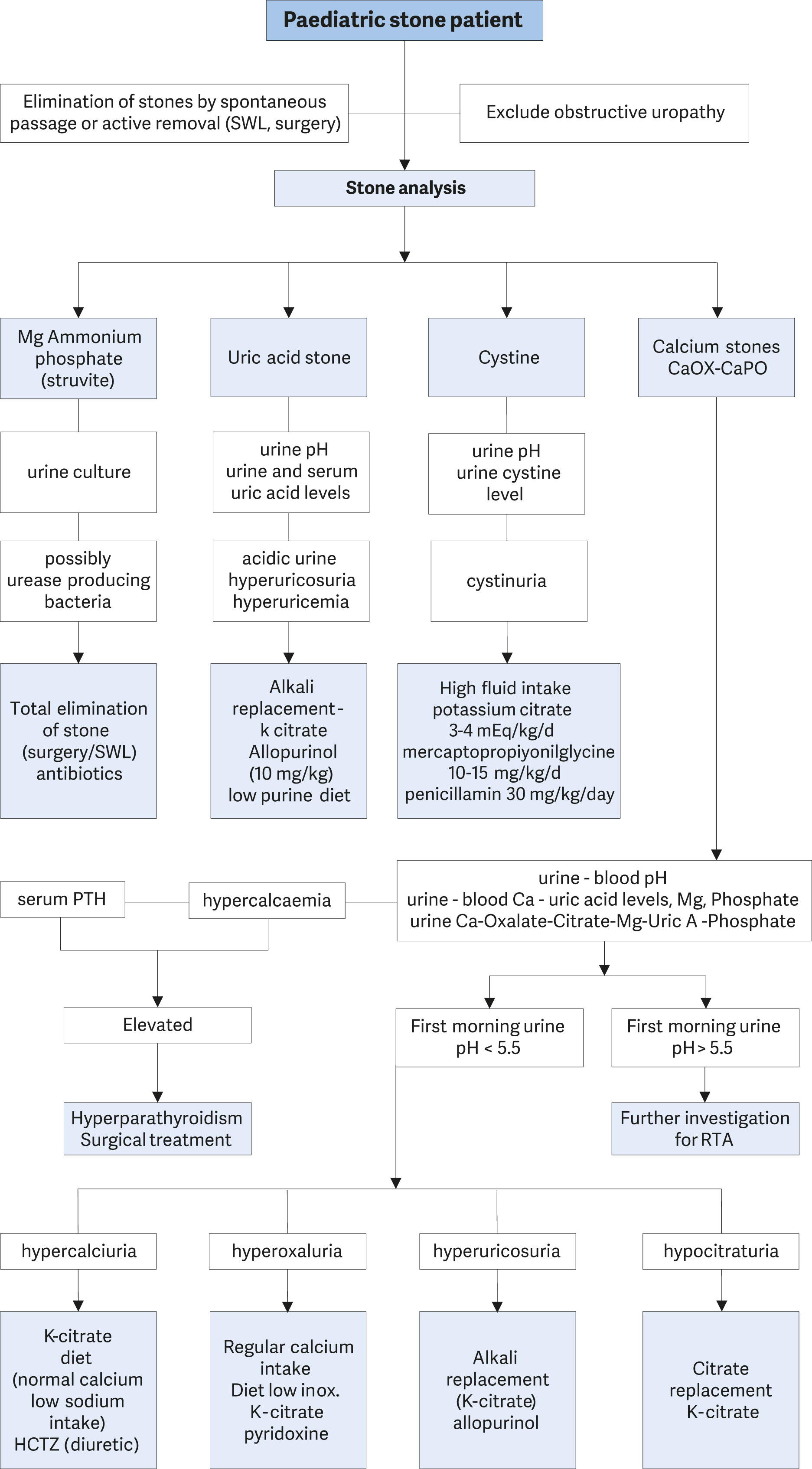 Ca = calcium; HCTZ = hydrochlorothiazide; Mg = magnesium; Ox = oxalate; PTH = parathyroid hormone;SWL = extracorporeal shockwave lithotripsy; RTA = renal tubular acidosis; Uric A = uric acid.
Ca = calcium; HCTZ = hydrochlorothiazide; Mg = magnesium; Ox = oxalate; PTH = parathyroid hormone;SWL = extracorporeal shockwave lithotripsy; RTA = renal tubular acidosis; Uric A = uric acid.
3.15.3.3. Urolithiasis in infants
Approximately 9 to 23% of paediatric urolithiasis patients are under one year old. Infantile urolithiasis appears to be a separate entity since the aetiology and the clinical course of the disease is different than in other age groups. A study on 2,513 children with urolithiasis demonstrated that microlithiasis (< 3mm) in infants should be differentiated from other age groups since the majority of them (85%) resolve spontaneously after one year of follow-up. It has also been shown that underlying metabolic abnormality is different than in older children. In this specific age group, calcium oxalate stones are not as common as in older age groups, whereas ammonium acid urate stones are more common [1011,1057]. However if the stone size increases or the patient becomes symptomatic during follow-up, it should be treated appropriately. Another study found that only 15% of infantile urolithiasis required intervention after one year follow-up and the only predictor for intervention was the size of the stone [1058]. Two other studies concluded that stone size larger than 4.5 mm and 5 mm in infants are more likely to require intervention [1059,1060]. Therefore observation should be the primary option for the majority of the infantile urolithiasis; if the patient becomes symptomatic or there is an increase in size, intervention can be discussed.
If an intervention is planned, SWL, retrograde intra-renal surgery (RIRS) or percutaneous nephrolithotomy (PCNL) can be offered depending on the characteristics of the stone and the patient. All treatment modalities were found to be feasible with high success rates in infants [1061-1063].
3.15.4. Management
Adequate fluid intake and restricting the use of salt within daily allowance range are the general recommendations besides the specific medical treatment against the detected metabolic abnormalities. With the advance of technology, stone management has changed from open surgical approaches to endoscopic techniques that are less invasive. Deciding on the type of treatment depends on the number, size, location, stone composition and the anatomy of the urinary tract [1053,1064,1065]. Expectant management is the initial management in children with asymptomatic small size stones (< 4-5 mm ) with a possibility of spontaneous clearance. A study in a paediatric population showed that stone size > 6.7mm and haematuria were negative predictors for spontaneous stone passage [1066]. There is no consensus on the size of stones for different ages eligible for clearance and the duration of conservative follow-up. Adult literature reveals the benefits of medical expulsive therapy (MET) using α-blockers. Although, experience in children is limited showing different results [1067], a meta-analysis of three randomised and two retrospective studies demonstrate that treatment with MET results in increased odds of spontaneous ureteral stone passage and a low rate of adverse events [1054,1068]. Stone size, and ureteral wall thickness were found to be highly predictive for MET success; patient age, BMI, stone density and degree of hydronephrosis had no predictive value in this aspect [1069]. Another RCT in the age group of 6-14 years comparing the effectivity of Silodosin, Tamsulosin and placebo as MET for distal ureteric stones less than 1 cm revealed higher stone expulsion rate for Silodosin (89.3%), compared to Tamsulosin (74.5%) and placebo (51.8%) in children [1070]. A Cochrane review including 125 children from 1-18 years old with Ca-containing idiopathic nephrolithiasis showed that oral potassium citrate may reduce recurrence after SWL; however a substantial number of children stopped medication due to adverse events [1071].
Currently, most paediatric stones can easily be managed by SWL, RIRS or PCNL. Only a small portion of children with anatomical abnormalities may require other types of surgical intervention (open, robotic, laparoscopic). All attempts must be made to completely remove all stones since post-operative residual fragments pass spontaneously in only 20-25% of cases [1072,1073]. A congenital obstructive uropathy should be managed together with stone removal therapy to prevent recurrence.
3.15.4.1. Extracorporeal shockwave lithotripsy
Many reports confirm that SWL can be performed in children with no suspicion of long-term morbidity of the kidney [1074-1081].
The mean number of shockwaves for each treatment is approximately 1,800 and 2,000 (up to 4,000 if needed) and the mean power settings vary between 14 kV and 21 kV. Recently, two separate RCTs compared the outcomes of low vs. intermediate frequency during SWL and found no significant difference [1082,1083]. The use of US and digital fluoroscopy has significantly decreased the radiation exposure and it has been shown that children are exposed to significantly lower doses of radiation compared to adults [1064,1084,1085]. Concerns about anaesthesia no longer present a problem due to advances in technique and medication, even in the infant age group. The type of anaesthesia should be general or dissociative for children under ten years of age, whereas conventional intravenous sedation or patient-controlled analgesia is an option for older children who are able to co-operate [1086] (LE: 2b). The general perception of paediatric SWL requiring anaesthesia has been challenged by a study showing that SWL without anaesthesia can be performed safely with comparable success rates in co-operative children > 9 years of age [1087].
Stone-free rates are significantly affected by various factors. Regardless of the location, as the stone size increases, the stone-free rates decrease and retreatment rate increases. The stone-free rates for < 1 cm, 1-2 cm, > 2 cm and overall, were reported as nearly 90%, 80%, 60% and 80%, respectively. As the stone size increases, the need for additional sessions increases [1064,1084,1085,1088-1092]. Previous history of open surgery also decrease the success of SWL [1093].
Localisation of the calculi has been described as a significant factor affecting the success rates in different studies. Stones in the renal pelvis and upper ureter seem to respond better to SWL. For these locations, the stone clearance rates are nearly 90%. However, SWL was found to be less effective for caliceal stones; particularly the lower caliceal stones. Several studies reported stone-free rates for isolated lower caliceal stones varying between 50% and 62% [1092,1094,1095].
Shockwave lithotripsy can also be used to treat ureteral calculi. However, this is a more specific issue and controversial. The success rates with SWL are less for distal ureteric stones. There may also be technical problems with localisation and focusing of ureteric stones in children [1092,1095-1097].
The type of machine used significantly influences success rates and complications. First-generation machines can deliver more energy to a larger focal zone, resulting in higher fragmentation rates in a single therapy. However, general anaesthesia is usually required due to the intolerable discomfort associated with a first-generation machine. Later-generation machines have a smaller focal zone and deliver less energy, and have a lower risk of pulmonary trauma, however, additional treatments may be needed. The success rate is higher in younger children [1090].
Although stenting does not affect stone clearance, overall complication rates are higher and hospital stay is longer in the unstented patient with larger stones [1090,1092]. Stenting is essential in solitary kidneys undergoing SWL treatment. Children with a large stone burden have a high risk of developing Steinstrasse and urinary obstruction and should be followed more closely for the risk of prolonged urinary tract obstruction after SWL. Post-SWL stent or nephrostomy tube placement may be needed in prolonged obstruction [1043,1089].
The Hounsfield Unit (HU) of stone on non-contrast tomography has also been shown to be a predictive factor for success in children and SWL was found to be more successful in stones with HU less than 600 [1073] and 1,000 [1098]. Two nomogram studies revealed male gender, younger age, smaller stone size, single stone, non-lower pole localisation and negative history for previous intervention are favourable factors for stone clearance in paediatric SWL [1099,1100]. A comparative study reported that these two nomograms are independent predictors of stone-free rate following SWL in paediatric patients [1101]. A systematic review confirmed that those two nomograms have equal value in predicting outcomes of SWL in children [1102]. Although, the invention of miniaturised endoscopic instruments seems to reduce the importance and popularity of SWL, it has the advantage of not carrying the risk of certain complications related to endoscopic surgeries and also with less post-operative emergency visits, pain and anaesthetic sessions [1103,1104]. Complications arising from SWL in children are usually self-limiting and transient. The most common are:
- renal colic;
- transient hydronephrosis;
- dermal ecchymosis;
- UTI;
- formation of Steinstrasse;
- sepsis;
- rarely, haemoptysis.
In children with sterile pre-operative urine cultures, antibiotic prophylaxis to decrease infectious complications is not recommended [1105]. However, every effort should be made to sterilise the urine before performing SWL, ureteroscopy (URS), or PCNL.
3.15.4.2. Percutaneous nephrolithotomy
Shockwave lithotripsy is the first choice for treating most renal paediatric stones. However, percutaneous renal surgery should be used for larger and complex stones. Pre-operative evaluation, indication and surgical technique are similar in children and adults. In most cases, PCNL is used as monotherapy, but is also used as an adjunctive procedure to other therapies.
The use of adult-sized instruments, in association with an increased number of tracts and sheath size, seems to increase blood loss. However, the development of small-calibre instruments (miniPCNL, ultraminiPCNL, superminiPCNL and microperc) means that PCNL can be used in children. Miniaturised PCNL has several advantages compared to standard PCNL, such as a smaller skin incision, single-step dilation and sheath placement, good working access for paediatric instruments, variable length, and lower cost [1105-1107].
As monotherapy, PCNL is considerably effective and safe. The reported stone-free rates in the recent literature are between 86.9% and 98.5% after a single session. These rates increase with adjunctive measures, such as second-look PCNL, SWL and URS. Even in complete staghorn cases, a clearance rate of 89% has been achieved following a single session [1108-1113]. The mean post-operative hospital stay is between one to four days and is much shorter than open surgery [1114]. The less invasive nature of this technique has made it superior to open surgery for treating renal stones in children [1115-1122].
The most frequently reported complications of PCNL in children are bleeding, post-operative fever or infection, and persistent urinary leakage. Bleeding requiring transfusion is reported in less than 10% [1118,1121,1123-1126] and is closely associated with stone burden, operative time, sheath size and the number of tracts [1118,1119,1127]. In recent studies, post-operative infectious complications, such as fever with or without documented UTI, are reported as less than 15% [1118,1121,1123,1124,1126,1128] and the origin of fever is not always found to be the infection. Due to the smaller size of the probes, laser energy is easier to use in smaller instruments and is more useful for paediatric cases [1108,1129-1131].
Using high power laser (> 40 W) during PCNL is feasible and may be helpful in the treatment of staghorn stones [1132], but it should be kept in mind, that increased temperatures inside the smaller paediatric kidney might lead to tissue damage, which has been shown in simulation models [1133].
With the availability of smaller size instruments, miniaturised PCNL (‘miniperc’) through a 13F or 14F sheath [1107,1128,1134] as well as ultramini-PCNL (UMP) through 12F sheaths [1135] have become possible, with decreased transfusion rates [1128]. The mini- and supermini-PCNL (SMP) were shown to have higher efficacy with low complication rates (< Clavien grade 3b) which were deemed to be a safe alternative to SWL by some authors [1115,1136]. In this study, 108 children under 12 years old with single stone (10-20mm) in the renal pelvis or calyces were randomised into two groups; either miniPCNL or SWL. Stone-free rate (SFR) after single session was significantly higher for PCNL (88.9%) compared to SWL (55.6%) [1115]. After second and third sessions, SWL success increased to 88.8% [1137]. The complication rates were 22.2% in PCNL and 14.8% in SWL without statistical significance.
The SMP was shown to be advantageous over mini-PCNL in terms of complications with similar stone-free rates [1137,1138]. This miniaturisation has been further developed into the technique of ‘micro-perc’ using a 4.85F ‘all-seeing needle’. This technique enables the stone to be fragmented by a laser in situ and left for spontaneous passage [1139]. A study revealed that microperc provides a similar SFR with similar complication rates and a lower additional treatment rate compared with SWL in the treatment of kidney stone disease in children [1140]. For stones 10-20 mm, micro-PCNL was shown to have comparable results, with less bleeding, compared to mini-PCNL [1116] and similar outcomes with less anaesthetic sessions compared to RIRS [1122]. As experience has accumulated in adult cases, new approaches have started to be applied in children, including tubeless PCNL. This technique has been used in uncomplicated surgery for stones < 2 cm, with patients left either with an indwelling catheter or double J stent in the ureter [1125,1141] or totally tubeless [1142]. Moreover, use of US for establishment of access is gaining popularity [1117,1120,1143].
Traditionally, PCNL in children is performed in prone position. Another trend in the literature is the performance of PCNL in flank-free modified supine position in children [1143,1144]. The proposed advantages are shorter operative time and enabling a simultaneous ureteroscopic procedure without changing the position of the patient. In a study, 55 paediatric patients with kidney stones who underwent UMP were randomised into two groups; flank-free modified supine position (FFMS) versus prone position. Stone free rates and complications rates were similar but the operative time was found to be shorter for supine position [1145].
For post-operative pain management, two RCTs showed that intercostal nerve block or erector spinae bloc were shown to provide effective post-operative analgesia in paediatric patients [1146,1147].
3.15.4.3. Ureterorenoscopy
The increasing availability of smaller size endourological equipment has made it possible to manage paediatric ureteral stones using endoscopic techniques.
The technique used in children is similar to the one used in adults. It is strongly recommended that guidewires are used and the procedure is performed using direct vision. Routine balloon dilation of the ureterovesical junction and ureteral stenting are controversial. In general, ureteric dilatation is being performed only in selected cases. There is a tendency to use hydrodilation more because it is similarly effective [1108,1148,1149].
Different lithotripsy techniques, including ultrasonic, pneumatic and laser lithotripsy, have all been shown to be safe and effective. Due to the smaller size of the probes, laser energy is easier to use in smaller instruments and is more useful for paediatric cases [1150].
All studies reporting the use of endoscopy for ureteric stones in children have clearly demonstrated that there is no significant risk of ureteric strictures or reflux with this mode of therapy. The risk of post-operative hydronephrosis depends on the presence of impacted stone and ureteral injury during operation [1151]. A multi-institutional study on the use of semi-rigid ureteroscopy for ureteral calculi in children showed that the procedure is effective with a 90% SFR and efficacy quotient. The study also focused on the factors affecting the complication rates. The authors found that, although operating time, age, institutional experience, orifice dilation, stenting and stone burden were significant on univariate analysis, multivariate analysis revealed that operating time was the only significant parameter affecting the complication rate [1148]. However, for proximal ureteral stones semi-rigid ureteroscopy is not a good first option because of higher complication and failure rates [1152].
A literature review contains a growing number of case series on the use of flexible ureterorenoscopic interventions in children. Both intrarenal and ureteric stones can be treated using this approach [1153-1158]. In these series, the authors generally did not use active orifice dilation, but attempted to use a ureteral sheath where possible. However, an important problem was the inability to obtain retrograde access to the ureter in approximately half of the cases [1154,1156]. This problem can be overcome by stenting and leaving the stent indwelling for passive dilation of the orifice, and performing the procedure in a second session. The success rates varied between 60 and 100%, with a negligible number of complications [1153,1155-1157,1159]. The need for additional procedures was related to stone size [1157]. Radiation exposure during URS can be minimised by using Flat Panel Detector c-Arms while simultaneously improving image quality [1160].
One RCT and four other comparative studies showed that RIRS had similar stone-free rates compared to SWL after three months, with fewer sessions [1103,1161-1164]. However for stones larger than 20 mm, RIRS monotherapy has lower stone-free rates than mini-PCNL with the advantages of decreased radiation exposure, fewer complications and shorter hospital stay [1165]. In contrast, for stones between 10-20 mm, RIRS has similar success and complication rates and shorter hospital stay and low radiation exposure when compared to micro-PCNL [1166]. A systematic review revealed that compared with the other two treatments, PCNL had a longer operative time, fluoroscopy time and hospital stay. Shockwave lithotripsy had a shorter hospital stay, higher retreatment rate and auxiliary rate in comparison with the other two treatments. It was also shown that PCNL presented a higher efficacy quotient than the other two treatments, and RIRS had a lower efficiency than SWL and PCNL. In the subgroup analysis of paediatric patients with stone < 20 mm, the comparative results were similar to those described above, except for the higher complication rate of PCNL than SWL [1167].
3.15.4.4. Open or laparoscopic stone surgery
Most stones in children can be managed by SWL and endoscopic techniques. However, in some situations, open surgery is inevitable. Good candidates for open stone surgery include very young children with large stones and/or a congenitally obstructed system, which also requires surgical correction. Open surgery is also necessary in children with severe orthopaedic deformities that limit positioning for endoscopic procedures.
In centres with well-established experience, a laparoscopic approach may be a good alternative for some cases as a last resort before open surgery. Suitable candidates include patients who have a history of previously failed endoscopic procedures, complex renal anatomy (ectopic or retrorenal colon), concomitant UPJ obstruction or caliceal diverticula, mega-ureter, or large impacted stones. Laparoscopic stone surgery via conventional or a robot-assisted transperitoneal or retroperitoneal approach can be attempted. However, there is limited experience with these techniques and they are not routine therapeutic modalities [1168-1171].
Bladder stones in children can usually be managed by endoscopic techniques. A recent randomised trial compared transurethral cystolithotripsy versus percutaneous cystolithotripsy for bladder stones smaller than 30 mm and found similar success and complication rates with success rates more than 95% [1172]. Open surgery may also be used for very large bladder stones or for bladder stones caused by an anatomical problem.
In addition to the advantages and disadvantages of each treatment modality for the specific size and location of the stone, consideration has to be given to the availability of the instruments and the experience with each treatment modality before the choice of technique is made. Recommendations for interventional management are given in Table 4.
Table 4: Recommendations for management in paediatric stones
Stone size and localisation* | Primary treatment option | Alternative treatment options | Comment |
Infant microlithiasis (<3mm, any location) | Observation | Intervention and/or medical treatment | Individualised decision according to size progression, symptoms and metabolic factors. |
Staghorn stones | PCNL | Open/SWL | Multiple sessions and accesses with PCNL may be needed. Combination |
Pelvis < 10 mm | SWL | RIRS/PCNL | |
Pelvis 10-20 mm | SWL/PCNL/RIRS | Multiple sessions with SWL may be needed. PCNL and RIRS have a similar recommendation grade. | |
Pelvis > 20 mm | PCNL | SWL/RIRS | Multiple sessions with SWL may be needed. |
Lower pole calyx < 10mm | Observation or SWL | PCNL/RIRS | Stone clearance after SWL is lower than other locations. |
Lower pole calyx > 10mm | PCNL | RIRS/SWL | Anatomical variations are important for complete clearance after SWL. |
Upper ureteric stones | SWL | URS | Flexible scopes may be needed in case of retropulsion. |
Lower ureteric stones | URS | SWL | |
Bladder stones | Endoscopic (transurethral or percutaneous) | SWL/Open | Open is easier and with less operative time with large stones. |
* Cystine and uric acid stones excluded.
PCNL = percutaneous nephrolithotomy; SWL = shockwave lithotripsy; RIRS = retrograde intrarenal surgery;URS = ureteroscopy.
3.15.5. Summary of evidence and recommendations for the management of urinary stones
Summary of evidence | LE |
The incidence of stone disease in children is increasing. | 2 |
Contemporary surgical treatment is based on minimally invasive modalities. Open surgery is very rarely indicated. | 2a |
The term ‘clinically insignificant residual fragments’ is not appropriate for children since most of them become symptomatic and require intervention. | 2b |
Majority of the kidney stones < 3 mm in infants resolve spontaneously. | 3 |
Recommendations | Strength rating |
Use plain abdominal X-ray and ultrasound as the primary imaging techniques for the diagnosis and follow-up of stones. | Strong |
Use low-dose non-contrast computed tomography in cases with a doubtful diagnosis, especially of ureteral stones or complex cases requiring surgery. | Strong |
Perform a metabolic evaluation in any child with urinary stone disease. Any kind of interventional treatment should be supported with medical treatment for the underlying metabolic abnormality, if detected. | Strong |
Limit open surgery under circumstances in which the child is very young with large stones, in association with congenital problems requiring surgical correction and/or with severe orthopaedic deformities that limit positioning for endoscopic procedures. | Strong |
Observe infant microlithiasis, unless symptoms occur or size increases significantly. | Strong |
3.16. Obstructive pathology of renal duplication: ureterocele and ectopic ureter
3.16.1. Epidemiology, aetiology and pathophysiology
Ureterocele and ectopic ureter are the two main anomalies associated with complete renal duplication, but they also occur in a single system. At present, antenatal US detects both conditions in the majority of cases if associated with obstruction, and diagnosis is confirmed after birth by further examination. Later in life, these anomalies are revealed by clinical symptoms: UTI, pain, calculus formation, disturbances of micturition, and urinary incontinence. There is a wide variation of symptoms in patients with ureterocele (from the asymptomatic patient to urosepsis, urinary retention and upper tract dilatation after birth).
3.16.1.1. Ureterocele
Ureterocele is four to seven times more frequent in female than in male patients; the overall incidence in autopsies is around one in 4,000 children. Around 80% is associated with the upper pole ureter in duplicated systems and 20% in single systems. About 10% of ureteroceles are bilateral [1173].
3.16.1.2. Ectopic ureter
Ectopic ureter is less frequent than ureterocele (10 in 19,046 autopsies), but is also more common in female patients (male to female ratio is 1:5). Some remain asymptomatic, therefore, the true incidence is difficult to determine [1174]. Eighty per cent of ectopic ureters are associated with complete renal duplication; however, in male patients about 50% of ectopic ureters are associated with a single system [1175]. The incidence of ectopic ureter is 3.5% in patients with anorectal malformations [1176].
3.16.2. Classification systems
3.16.2.1. Ureterocele
Ureterocele is a cystic dilatation that develops in the intravesical part of the submucosal ureter. The aetiology remains unclear [1177-1179]. A single-system ureterocele is associated with a kidney with one ureter, and in duplex systems, the ureterocele belongs to the upper pole.
Ureteroceles usually cause obstruction of the upper pole, but the degree of obstruction and functional impairment is variable according to the type of ureterocele and upper pole dysplasia. In the orthotopic form, there is often no or only mild obstruction, and frequently the function of the moiety is normal or slightly impaired, and the corresponding ureter may be dilated. Cystic renal dysplasia is also associated with a single system ureterocele [1180]. Vesicoureteral reflux can be observed in 50% on the ipsilateral side and 20% on the contralateral side. Reflux into the ureterocele is uncommon [1181]. In the ectopic form, the upper pole is altered, frequently dysplastic, and hypo-functional or non-functional [1182]. The corresponding ureter is a mega-ureter. In the caeco-ureterocele (see definition below), the upper pole of the renal duplication is dysplastic and non-functional. Histological evaluation demonstrated that the changes represent a process of maldevelopment and may not result from infections or obstruction [1182].
3.16.2.1.1. Ectopic (extravesical) ureterocele
If any portion of the ureterocele extends into the bladder neck or urethra, it is called an ectopic ureterocele. Ectopic ureterocele is the most common form of ureterocele (> 80%). It can be voluminous, dissociating the trigone and slipping into the urethra, and may prolapse through the urethral meatus (caeco-ureterocele). The ureterocele orifice is tight, and located in the bladder itself or below the neck. The ureter corresponding to the lower pole moiety is raised by the ureterocele and is frequently refluxing or compressed by the ureterocele, leading to an obstructive mega-ureter. A contralateral renal duplication is associated with 50% of cases. Occasionally, large ureteroceles are responsible for reflux or obstruction of the contralateral upper tract.
3.16.2.1.2. Orthotopic (intravesical) ureterocele
The intravesical or orthotopic ureterocele is completely located in the bladder. Intravesical ureteroceles are mostly combined with a single kidney system and account for about 15% of cases. It is diagnosed more in older children or adults.
3.16.2.2. Ectopic ureter
The term ectopic ureter describes a ureter with the orifice located at the bladder neck, in the urethra or outside the urinary tract. The ureter can drain the upper pole of a duplex or single system. There is a fundamental difference between the sexes. In boys, the ectopic orifice is never below the external sphincter.
In girls, the ureteral orifice may be located [1183]:
- in the urethra, from the bladder neck to the meatus (35%);
- in the vaginal vestibule (34%);
- in the vagina (25%);
- in the uterus and Fallopian tube (6%).
In boys, the ureteral orifice may be located [1183]:
- in the posterior urethra (47%);
- in the prostatic utricle (10%);
- in the seminal vesicles (33%);
- in the vas deferens or ejaculatory ducts (10%).
3.16.3. Diagnostic evaluation
3.16.3.1. Ureterocele
Prenatal US easily reveals voluminous obstructive ureteroceles [1184]. In cases with a small upper pole or a slightly obstructive ureterocele, prenatal diagnosis is difficult. If prenatal diagnosis is impossible, the following clinical symptoms, besides incidental findings, can reveal the congenital anomaly at birth or later:
- At birth, a prolapsed and sometimes strangulated ureterocele may be observed in front of the urethral orifice. In a newborn boy, it might cause acute urinary retention, simulating urethral valves.
- The early symptom of pyelonephritis in either sex may lead to the diagnosis.
- Later symptoms can include dysuria, recurrent cystitis and urgency.
In cases of prenatal diagnosis, at birth US confirms the ureteral dilatation that ends at the upper pole of a renal duplication. It also demonstrates the presence of a ureterocele in the bladder, with a dilated ureter behind the bladder.
At this point, it is important to assess the function of the upper pole using nuclear renography of the region of interest. This is best assessed with DMSA, however this requires a careful systematic review of the images [1185]. Magnetic resonance urography may visualise the morphological status of the upper pole and lower moieties and of the contralateral kidney as well as it can detect renal scars [1186,1187]. Using functional MR urography, differential renal function can be assessed with low intra- and interobserver variability [1188]. Based on the prevalence of high-grade reflux, VCUG is mandatory for identifying ipsilateral or contralateral reflux and assessing the degree of intra-urethral prolapse of the ureterocele [1189]. Urethrocystoscopy may reveal the pathology in cases where it is difficult to make the differential diagnosis between ureterocele and ectopic mega-ureter.
3.16.3.2. Ectopic ureter
Most of the ectopic mega-ureters are diagnosed primarily by US. In some cases, clinical symptoms can lead to diagnosis:
- In neonates: dribbling of urine, pyuria, and acute pyelonephritis.
- In young girls: permanent urinary incontinence besides normal voiding, or significant vaginal discharge as the equivalent of incontinence; an ectopic orifice may be found in the meatal region [1190].
- In pre-adolescent boys: epididymitis is the usual clinical presentation and the seminal vesicle may be palpable.
Ultrasound, radionuclide studies (DMSA, VCUG, MR urography, high-resolution MRI, and cystoscopy) are the diagnostic tools to assess function, to detect reflux and rule out ipsilateral compression of the lower pole and urethral obstruction [1191]. In some cases, the large ectopic ureter presses against the bladder and can look like a pseudo-ureterocele [1192].
Girls who present with life-long minimal urinary incontinence, never being dry, normal bladder function, complete emptying, and normal US are very suspicious for ectopic ureter. This needs to be excluded or confirmed by MRI as it is the most sensitive method [1193].
3.16.4. Management
3.16.4.1. Ureterocele
Management is controversial with a choice between a non-operative approach, endoscopic decompression, ureteral re-implantation, partial nephroureterectomy, or complete primary reconstruction [1194-1199]. The choice of a therapeutic modality depends on the following criteria: clinical status of the patient (e.g. urosepsis); patient age; function of the upper pole; presence of reflux or obstruction of the ipsilateral or contralateral ureter; presence of bladder neck obstruction caused by ureterocele; intravesical or ectopic ureterocele; and caregivers’ and the surgeon’s preferences [1199]. When the diagnosis is made by US, prophylactic antibiotic treatment maybe indicated until a VCUG is performed.
3.16.4.1.1. Early treatment
In the presence of febrile infection or obstruction at the bladder neck, immediate endoscopic incision or puncture of the ureterocele is recommended. In a clinically asymptomatic child with a ureterocele and a non or hypofunctional upper pole, without significant obstruction of the lower pole and without bladder outlet obstruction, prophylactic antibiotic treatment is given until follow-up procedures are instigated. Decompression of the dilated system facilitates later reconstructive surgery [1200,1201].
3.16.4.1.2. Re-evaluation
Active surveillance is an option for antenatally detected ureteroceles, but long-term follow-up is necessary [1202]. Conservative treatment may be adopted in asymptomatic patients without any bladder outlet obstruction, severe hydroureteronephrosis of the ureterocele moiety or high-grade (over grade III) reflux [1199,1203]. A meta-analysis showed that, after primary ureterocele-incision, the re-operation rate is higher in those with an ectopic ureterocele compared to those with an intravesical ureterocele [1195]. Secondary surgery is necessary if decompression is not effective, significant reflux is present, or there is obstruction of the ipsi- or contralateral ureters, and/or bladder neck obstruction or retained ureterocele [1204].
Surgery may vary from upper pole nephrectomy to complete unilateral LUT reconstruction [1198,1205-1207]. In an ectopic ureterocele with severe hydroureteronephrosis and without reflux, the primary upper tract approach without endoscopic decompression (partial upper-pole nephroureterectomy, pyelo/ureteropyelo/ureterostomy and upper-pole ureterectomy) has an 80% chance of being the definitive treatment [1199,1208]. Also a LUT approach in those with a poorly or non-functioning upper pole is an option [1209]. Today, despite successful surgery, some authors think, that surgery may not be necessary at all in some patients [1210], as less aggressive surgical treatment and non-operative management over time can achieve the same functional results [1211]. There is emerging evidence on minimally invasive surgical approach (laparoscopic and robot assisted) for upper pole nephrectomy with similar operating time to open surgery [1212,1213].
Figure 11: Algorithm for the management of duplex system ureteroceles after the first 3-6 months of life [1214]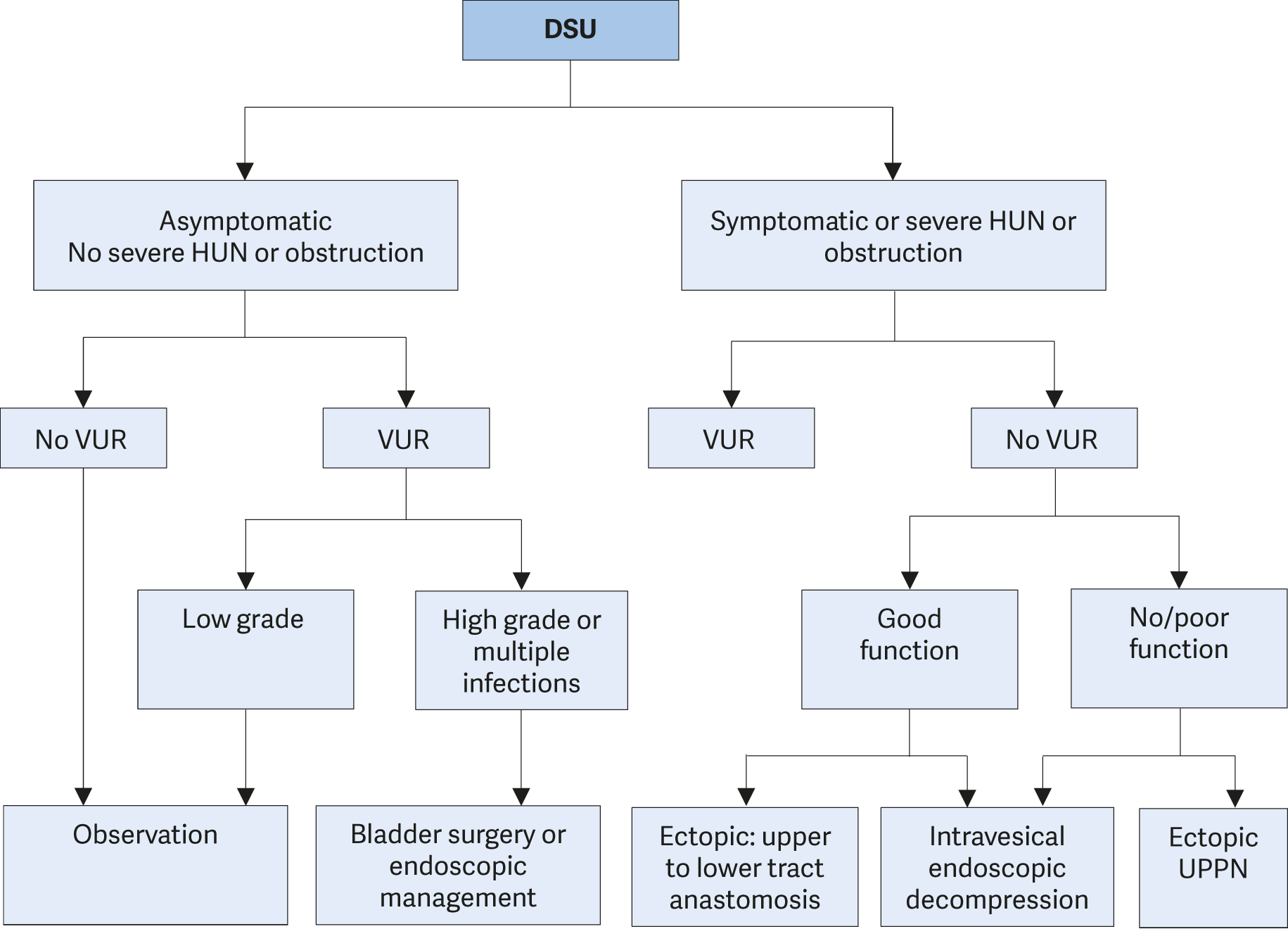 DSU = duplex system ureterocele; HUN = hydroureteronephrosis; UPPN = upper pole partial nephrectomy;VUR = vesicoureteric reflux to the lower pole.
DSU = duplex system ureterocele; HUN = hydroureteronephrosis; UPPN = upper pole partial nephrectomy;VUR = vesicoureteric reflux to the lower pole.
Obstruction is considered to be the presence of non-refluxing dilatation of non-ureterocele-bearing moieties (especially of the lower pole) or of an obstructive drainage pattern on diuretic renography.
3.16.4.2. Ectopic ureter
In the majority of cases, the upper pole is dysplastic and poorly functioning. There are a variety of therapeutic options, each with its advantages and disadvantages. In non-functioning moieties with recurrent infections, heminephro-ureterectomy is a definite solution. Ureteral reconstruction (ureteral re-implantation/ ureteroureterostomy/ureteropyelostomy and upper-pole ureterectomy) are other therapeutic options especially in cases in which the upper pole has function worth preserving. These procedures can be performed through an open laparoscopic or robotic assisted approach [1213,1215-1217]. So far there is no superior approach [1218]. In patients with bilateral single ectopic ureters (a very rare condition), an individual approach depending on the sex, renal and bladder function of the patient is necessary. Usually the bladder neck is insufficient in these patients [1219].
3.16.5. Summary of evidence and recommendations for the management of obstructive pathology of renal duplication: ureterocele and ectopic ureter
Summary of evidence | LE |
Ureterocele and ectopic ureter are associated with complete renal duplication, but they also occur in a single system. | 1 |
In most cases, in young children (first years of life) diagnosis is done by US. | 1 |
In older children clinical symptoms will prompt assessment. | 1 |
Management includes a conservative approach, endoscopic decompression, partial nephroureterectomy, or complete primary reconstruction. Choice of treatment will depend on: clinical status of the patient (e.g., urosepsis); patient age; function of the upper pole; presence of reflux or obstruction of the ipsilateral or contralateral ureter; presence of bladder neck obstruction caused by ureterocele; intravesical or ectopic ureterocele. | 3 |
Recommendations | LE | Strength rating | ||
Ureterocele | Diagnosis | Use ultrasound (US), radionuclide studies (mercaptoacetyltriglycine (MAG3)/ dimercaptosuccinic acid (DMSA)), voiding cystourethrography (VCUG), magnetic resonance urography, high-resolution magnetic resonance imaging (MRI), and cystoscopy to assess function, to detect reflux and rule out ipsilateral compression of the lower pole and urethral obstruction. | 3 | Weak |
Treatment | Select treatment based on symptoms, function and reflux as well on surgical and parenteral choices: observation, endoscopic decompression, ureteral re-implantation, partial nephroureteretomy, complete primary reconstruction. Offer, early endoscopic decompression to patients with an obstructing ureterocele. | 3 | Weak | |
Ectopic ureter | Diagnosis | Use US, DMSA scan, VCUG or MRI for a definitive diagnosis. | 3 | Weak |
Treatment | Treatment In non-functioning moieties with recurrent infections, heminephro-ureterectomy is a definitive solution. Ureteral reconstruction (ureteral re-implantation/ureteroureterostomy/ ureteropyelostomy and upper pole ureterectomy) are other therapeutic option especially in cases in which the upper pole has function worth preserving. | 3 | Weak | |
3.17. Disorders of sex development
3.17.1. Introduction
Formerly called ‘intersex disorders’, this constellation of conditions has been the subject of a consensus document in which it was decided that the term ‘intersex’ should be changed to ‘disorders/differences of sex development’ (DSD), however the original term is still used in the resolution of the Parliamentary Assembly of the Council of Europe (see below) [1220].
The new classification has arisen due to advances in knowledge of the molecular genetic causes of abnormal sexual development, controversies inherent to clinical management, and ethical issues. Controversial and negative terminology, e.g., ‘pseudohermaphroditism’ and ‘hermaphroditism’, have been renamed according to new pathophysiological insights. Furthermore, some conditions presenting with severe male genital malformation, such as penile agenesis and cloacal exstrophy, which could not previously be categorised, have now also been included. The term ‘disorders/differences of sex development’ is proposed to indicate congenital conditions with atypical development of chromosomal, gonadal or anatomical sex.
In addition to this, in 2017 the Parliamentary Assembly of the Council of Europe decided on a resolution termed: “Promoting the human rights of and eliminating discrimination against intersex people” [1221]. The Assembly concluded that the majority of ‘intersex’ people (cited verbatim from the resolution) were physically healthy and that only a few suffered from medical conditions that put their health at risk. Furthermore, they stated that the prevailing medical view at that time was that the bodies of ‘intersex’ children could, and should be made to conform to either a male or a female paradigm, often through surgical and/or hormonal intervention, and that this should be performed as early as possible so that these children could then be raised in the gender corresponding to their assigned sex. The Parliamentary Assembly considered that this approach involved serious breaches of physical integrity and autonomy, with many cases concerning very young children or infants who were unable to give informed consent and whose gender identity was unknown.
Therefore, the Parliamentary Assembly called on Council of Europe member states to effectively protect children’s rights to physical integrity and bodily autonomy, and to empowering ‘intersex’ people with the following rights: Medically unnecessary sex-“normalising” surgery, sterilisation and other treatments practised on ‘intersex’ children without their informed consent should be prohibited, and in addition that it has to be ensured that, except in cases where the life of the child is at immediate risk, any treatment that seeks to alter the sex characteristics of the child including their gonads, genitals or internal sex organs, is deferred until such time as the child is able to participate in the decision, based on the right to self-determination, and on the principle of free and informed consent.
The Panel refers to the consensus documents mentioned above as well as on the Parliamentary Assembly resolution. This chapter will focus on what is relevant for the practising paediatric urologist as the urologist is likely to be involved in neonates with DSD conditions.
Overall, evidence-based literature on DSD is sparse. There are no RCTs, and most studies are based on retrospective, clinical descriptive studies, or on expert opinion. An exception is made in relation to the risk of gonadal cancer, for which the level of evidence is higher [1222].
Disorders/differences of sex development can present as a prenatal diagnosis, neonatal diagnosis, or late diagnosis. Prenatal diagnosis can be based on karyotype or sonographic findings; a neonatal diagnosis is based on genital ambiguity, and a late diagnosis is usually made as a result of early or delayed puberty. In this guideline, the focus is on the neonatal presentation, where the paediatric urologist plays a more central role. There have been several publications over the last couple of decades exploring the role of prenatal corticosteroid treatment of patients with congenital adrenal hyperplasia. The Endocrine Society still proclaims their use to be restricted to research settings, and that this treatment remains experimental [1223,1224]. For late diagnoses, we refer to endocrinology and gynaecology guidelines on precocious and delayed puberty, where paediatric urologists play a less central role [1225,1226].
Dealing with neonates with DSD requires a multi-disciplinary approach, which should ideally include geneticists, neonatologists, paediatric and adult endocrinologists, paediatric urologists, gynaecologists, psychologists, ethicists and social workers. Each team member should ideally be experienced in DSD and a team should have treated enough patients to ensure experience.
A discrepancy is often perceived between research topics proposed by research scientists and those considered important by DSD patients [1227]. As a result of this discrepancy, collaborative networks such as the ‘dsd-LIFE’ consortium, have been established to include research scientists, health professionals, patient families, and support groups (available from: https://www.dsd-life.eu/home/index.html). In the dsd-LIFE study, there is a focus on what patients and care-givers consider to be a priority, and research is then carried out around that issue. In addition, the newly established European Reference Network (ERN) covering rare endocrine conditions (Endo-ERN) considers patient participation in research and database management to be crucial (available from: https://endo-ern.eu/.
3.17.2. International Consensus Statements on DSD Management
There have been four published consensus statements in relation to the investigations and management of DSD. In general, these statements have focused on the impact of DSD on older age groups, the importance of long-term prospective multi-disciplinary, multi-centre data collection with a focus on patient reported outcomes. The ultimate ambition is to preserve physical and psychological function in these future adults [1228]. The consensus proposal from European Society of Paediatric Radiology task force predominantly focused on imaging modalities and calls for the optimisation of US in initial and interval assessments of anatomy, with MR imaging and cystovaginography used as adjunctive modalities [1229]. The COST Action BM1303 working group 1 consensus statement from Europe raised the concern of the effects of delayed genital and gonadal surgery on physical, psychological, and sexual well-being, as well as the potential malignant risks of retained gonads. Support tools need to be developed to help guide affected families and children with a balance struck between surgery and the protection of human rights [1227]. The Canadian consensus statement broadly concurs with the above but differs slightly from their European counterparts. It suggests that sex assignment need not take place at birth, and there should be a recognition of the harms caused in the past by a paucity of information to parents, and that decisions involving surgery should take place involving a shared decision model. This consensus finally suggests that data is insufficient to determine the correct timing of surgery [1230].
3.17.3. Current classification of DSD conditions
There have been a number of published updates since the International Consensus Conference on ‘intersex’ and its subsequent publications on the classification of the various conditions of DSD. The latest of these was published by the Global DSD Update Consortium in 2016 [1231]. As the field of DSD is continuously developing, and knowledge and viewpoints change over time, an effort has been made to to consider diversity, inclusion, and equality, and therefore representatives from support and advocacy groups continue to be invited, with an aim to focus on patient care and the best possible quality of life.
According to the international consensus in 2005, DSDs have been defined as congenital conditions within which the development of chromosomal, gonadal and/or anatomic sex is atypical. The changes that were made according to terminology are as follows:
46XX DSD – This was formerly termed female pseudohermaphrodite, over-virilisation of an XX female, and masculinisation of an XX female. In this group the vast majority is due to classic congenital adrenal hyperplasia (CAH) with various degrees of masculinisation. Among all DSD conditions together, 46XX CAH patients comprise approximately 80% cases. These conditions are extremely important since they can be potentially life threatening days after birth due to a salt-loss phenomenon, and immediate medical care is mandatory.
46XY DSD – Previously termed male pseudohermaphroditism, undervirilisation of an XY male, and undermasculinisation of an XY male. This group is often quite heterogenous and includes the partial androgen insensitivity syndrome (PAIS), as well as the complete androgen insensitivity syndrome (CAIS) formerly called testicular feminisation.
Sex chromosome mosaicism DSD (45X; 45X/46XY; 47XXY) – This cohort consists of multiple variants with the mixed gonadal dysgenesis being the most important one. Many have a normal male phenotype and others may have asymmetric genitalia. One scrotal half often contains a gonad which is likely to be a testis whereas the other side is more in keeping with a labia majora usually with no palpable gonad, which will most likely be a streak gonad.
Ovotesticular DSD – This was previously described as a ‘true hermaphrodite’ because of the presence of ovarian and testicular tissue co-existing in the same individual meaning. There is great variability in phenotype with uni- or bilateral undescended gonads, which can present as one ovary and one testis, or as one or two ovotestes.
Non-hormonal/non-chromosomal DSD – This cohort was introduced as well, including newborns with cloacal exstrophy where bladder and intestines are exposed through a midline mesenchymal defect resulting from the failure of the clocal membrane to retract, which then rutpures. Others in this cohort include patients with aphallia, and severe micropenis. The latter one is a normally formed penis with a stretched length of < 2.5 standard deviation below the mean [1220,1232]. Micropenis should be distinguished from buried and webbed penis, which are usually of normal size. The length of the penis is measured on the dorsal aspect, while stretching the penis, from the pubic symphysis to the tip of the glans [1220].
3.17.4. Diagnostic evaluation
3.17.4.1. The neonatal emergency
The first step is to recognise the possibility of DSD (Table 5) and to refer the newborn baby immediately to a tertiary paediatric centre, fully equipped with neonatal, genetics, endocrinology and paediatric urology units. A diagnosis of a 46XX DSD as a result of congenital adrenal hyperplasia (the most common form of DSD) should not be delayed, and represents a neonatal emergency situation due to the possibility of salt loss which can be fatal.
Table 5: Findings in a newborn suggesting the possibility of DSD (adapted from the American Academy of Pediatrics)
Apparent male |
Severe hypospadias associated with bifid scrotum |
Undescended testis/testes with hypospadias |
Bilateral non-palpable testes in a full-term apparently male infant |
Apparent female |
Clitoral hypertrophy of any degree, non-palpable gonads |
Vulva with single opening |
Indeterminate |
Ambiguous genitalia |
3.17.4.2. Family history and clinical examination
A careful family history must be taken followed by a thorough clinical examination including various laboratory tests and imaging modalities (Table 6).
Table 6: Diagnostic work-up of neonates with DSD
History (family, maternal, neonatal) |
Parental consanguinity |
Previous DSD or genital anomalies |
Previous neonatal deaths |
Primary amenorrhoea or infertility in other family members |
Maternal exposure to androgens |
Failure to thrive, vomiting, diarrhoea of the neonate |
Physical examination |
Pigmentation of genital and areolar area |
Hypospadias or urogenital sinus |
Size of phallus |
Palpable and/or symmetrical gonads |
Blood pressure |
Investigations |
Blood analysis: 17-hydroxyprogesterone, electrolytes, LH, FSH, TST, cortisol, ACTH |
Urine: adrenal steroids |
Genetics: karyotype, next-generation sequencing-based molecular diagnostics, WES |
Ultrasound |
Genitogram |
hCG stimulation test to confirm presence of testicular tissue |
Androgen-binding studies |
Endoscopy |
ACTH = adrenocorticotropic hormone; FSH = follicle-stimulating hormone; hCG = human chorionic gonadotropin; LH = luteinising hormone; TST = testosterone; WES = whole exome sequencing
A thorough and standardised clinical examination in a neonate presenting with ambiguous genitalia is important. In addition to an accurate description of the ambiguous genitalia, detailed information should be documented on the palpability and localisation of the gonads. Data gathered through the various examinations described below should help the team to come to a final diagnosis. Medical photography can be useful, however this requires sensitivity and consent [1233].
Palpable gonad: If it is possible to feel a gonad, it is most likely to be a testis; this clinical finding therefore virtually excludes 46XX DSD.
Phallus: The phallus length, width, and glans width should be measured. A cotton bud placed at the suprapubic base of the implant of the stretched phallus allows for a good measurement of phallic length.
Urogenital sinus opening: The opening of the urogenital sinus must be well evaluated. A single opening has to be identified as well as a hymenal ring. Attention needs to be paid to the fusion of the labioscrotal folds as well as whether they show rugae or some discolouration.
Ultrasound can help to describe the palpated gonads or to detect non-palpable gonads [1229]. Mullerian structures like the vagina or utricular structures can be evaluated as well [1234,1235].
Genitography can provide some more information on the urogenital sinus, especially on the exact position of the confluence. Moreover, it gives evidence of possible duplication of the vagina.
Invasive diagnostics under general anaesthesia can be helpful in some cases. During genito-cystoscopy, the urogenital sinus can be evaluated as well as the level of confluence. It allows also for evaluation of the vagina or utriculus, the possible presence of a cervix at the top of the vagina.
Laparoscopy is necessary to obtain a final diagnosis on the presence of impalpable gonads and on the presence of Mullerian structures. If indicated, a gonadal biopsy can be performed [1236,1237].
Genetics has an increasing role in the diagnostic process of DSD. Karyotyping is usally at the beginning of the diagnostic process. Although next-generation sequencing-based molecular diagnostics and whole exome sequencing (WES) are becoming the gold standard for genetic evaluation, it may be difficult to prove variant causality or relate the genotype to the clinical presentation [1238].
These investigations will help to distinguish between various conditions of DSD, and provide a rapid diagnosis of congenital adrenal hyperplasia (CAH).
3.17.5. Gender assignment
In the current climate, it goes without saying that open, honest, and complete communication with caregivers and eventually the affected person is mandatory. Educational and psychological support regarding the impact is needed for each individual to make sense of their condition, relate to their community, and establish relationships. The lack of outcome data and different preferences make it challenging to determine whether and when to pursue gonadal or genital surgery. Shared decision making is critical, combining expert healthcare knowledge and the right of a patient or caregivers to make fully informed decisions. This entails a process of education, sharing of risks/benefits, articulating the uncertainties in DSD care and outcomes in addition to providing time for the patient and family to articulate back the risks and benefits of each option. The goal of all involved should be to individualise and prioritise each patient.
However, prior published adverse outcomes have led to recommendations to delay unnecessary surgery to an age when the patient can give informed consent. Surgery that alters appearance is not considered urgent. In 2017 the Parliamentary Assembly of the Council of Europe, the European Society for Paediatric Urology (ESPU) as well as the Societies for Pediatric Urology have taken a position in the debate on surgery for DSD [1221,1239,1240]. In an open letter to the Council of Europe, the European Society for Paediatric Urology, expressed its attitude to the above mentioned resolution and concentrated on a worrying issue dealing with medicosurgical care for children with DSD. It states that surgical interventions in children with DSD only being applied in emergency conditions is discordant with the definition of health according to the WHO, stating that health is not merely the absence of disease, but is a much broader concept, including physical, mental, and social domains. This especially applies to children, as favourable physical, social and emotional conditions are all critical factors for their optimal growth and development, which enables them to reach their full potential at an adult age. As social and emotional interactions with the parents or caregivers, being the most important adults in a young child’s life, form the basis for their future, treatment of children with DSD can best be organised in a patient- and family-centred multi-disciplinary setting, in an atmosphere based on openness, commitment and trust. Physicians, who daily take care of children with a variety of congenital conditions, the same as their parents or caregivers, are committed to the current as well as the future health and well-being of all children entrusted to their care. In contrast to what is alleged in the recommendation, parents and caregivers implicitly act in the best interest of their children and should be respected as their outstanding representatives, and should not be put aside by claiming prohibition regulations regarding the well-informed decisions they make on their behalf. Finally, in a published open letter, the ESPU advocate keeping dialogue open with professionals active in specialised centres for multi-disciplinary patient- and family-centred care as well as with patient societies, for which the present resolution is recognised as being a solid starting base [1241].
Genital surgery
The decision to proceed with genital surgery is acknowledged to be controversial. Patient-reported outcomes from adult patients who previously underwent early genital surgery demonstrate considerable variation, with perspectives dependent on, but not limited to, diagnostic category, gender, prior experience with surgical procedures, and contact with support groups [1242].
The majority of patients who have undergone surgery rated their appearance as satisfactory from an anatomical perspective. However, functional results were found to be less satisfactory due to the development of vaginal stenosis, or diminished sensation in the clitoris or the glans penis [1243]. Clinical decision-making with respect to genital surgery in patients with a DSD should not be made wantonly, but advisedly, in a patient and family-centred multi-disciplinary setting, on a case-by-case basis. These decisions should be supported and audited by improving information on long-term outcomes, informed consent, and contact with support groups at both an individual and an institutional level.
3.17.6. Risk of tumour development
The dysgenetic gonads of individuals with DSD have an increased risk of developing germ cell neoplasia in situ (GCNIS), previously known as carcinoma in situ, and overt germ cell cancer (GCC) as compared to the general population [1244]. The highest prevalence of GCC is seen in conditions characterised by disturbed gonadal development and in the presence of the Y chromosome or parts thereof (SRY & GBY encompassing regions) [1245]. In a large dsd-LIFE study the overall prevalence of neoplastic lesions was 12%. Subanalysis demonstrated a significantly higher prevalence of 36% in patients with 46 XY gonadal dysgenesis as compared with other DSD subtypes [1246]. Conversely, patients with testosterone biosynthesis disorders and androgen action disturbances (46XY DSD group) have a much lower risk (1-15%) for GCNIS development during childhood and had a limited tendency towards invasive progression of the lesions. It has been hyposthesised that a certain level of testosterone activity seems to be needed for GCNIS to progress to overt malignancy [1247,1248]. An overview of the risks of malignancy in different subtypes of DSD is shown in Table 7.
The issue of whether gonads should be removed and the timining of such surgery remains controversial and has been altogether questioned in some forms of DSD. Patients with, for example, CAIS benefit from the presence of testicles and the resultant aromatisation of the naturally occuring testosterone to oestrogens. The risk of malignant gonadal transformation in this subcategory is low (1.5%) with cases of malignancy first appearing after the second decade of life, thus allowing for the safe deferal of gonadectomy until after puberty [1248,1249]. This is however less clear for other subtypes of DSD, and needs to be assessed for each case according to several factors such as patient age, underlying DSD subtype and especially the presence of a Y chromosome. In such cases, the location of the gonad, possible fertility, hormonal potential of the gonad and the possibility of gonadal monitoring together with surgical/anaesthesic risks incurred by gonadectomy should be taken into account [1222,1241]. In general, intra-abdominal gonads have to be brought down to a superficial position or pexied to the abdominal wall to allow for monitoring, self examination and ultrasound guided biopsies. High-risk gonads that fail to be brought down, or streak-like gonads should be removed based on a risk- benefit analysis, and after appropriate inter-disciplinary review [1222,1241]. Biopsies should be reviewed by an experienced pathologist and specialised immunohistochemistry is recommended for measurement of expressions of PLAP and octamer-binding transcription factors 3 and 4, as it may be difficult to differntiate between GCNIS and delayed germ cell maturation in infants. Non-invasive markers such as serum microRNA (miRNA) for early-stage malignancy detection, have been developed, but have yet to be implemented in clinical practice [1222,1238].
Table 7: Risk of malignancy in different subtypes of DSD (Adapted from Looijenga et al., )
Risk | DSD | Malignancy risk (%) |
High | Gonadal dysgenesis, with Y, abdominal gonad PAIS non-scrotal gonad Frasier syndrome Denys-Drash with Y | 15-35 50 60 40 |
Intermediate | Turner syndrome with Y 17β- hydroxysteroid dehydrogenase deficiency Gonadal dysgenesis with Y PAIS scrotal gonad | 12 28 Unknown Unknown |
Low | Complete androgen insensitivity syndrome Ovotesticular DSD Turner syndrome without Y | 2 3 1 |
No | 5-Alpha Reductase Deficiency Leydig cell hyperplasia | 0 0 |
3.17.7. Quality of life
In general, adult patients with DSD report good quality of life and physical health, however there is an increased risk for both somatic and psychiatric morbidities [1251]. Furthermore, a lower quality of life was reported in the domain “social relationships”, which relates to personal relationships and sexual health [1252,1253]. In addition, patients with DSD report higher levels of psychological distress and mental health problems [1254,1255]. These elements should be included in the multi-disciplinary and holistic health care for these patients.
A person’s experienced gender is a fundamental aspect of one’s sense of self. Gender incongruence can occur when there is incongruence between the physical and experienced gender, and if this causes significant distress it furfills the criteria for the diagnosis gender dysphoria, according to the “Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)” of the American Psychiatric Association [1256]. Gender dysphoria is reported low in women with CAH, CAIS and complete gonadal dysgenesis favoring female sex of rearing. Gender dysphoria is reported high in females with 5-a reductase deficiency and 17β-Hydroxysteroid dehydrogenase-3 deficiency. Gender dysphoria is reported variable in PAIS or mixed gonadal dysgenesis [1257]. Approximately 3% of DSD patients undergo a gender change after puberty, which is a small group, but larger when compared to the general population [1258,1259].
3.17.8. Recommendations for the management of disorders/differences of sex development
Recommendations | Strength rating |
Do not delay diagnosis and treatment of any neonate presenting with ambiguous genitalia since salt-loss in a 46XX CAH girl can be fatal. | Strong |
Refer children to experienced centres where neonatology, endocrinology, (paediatric) urology, psychology and transition to adult care are guaranteed. | Strong |
Utilise a multi-disciplinary approach and a shared decision model in patients with DSD conditions including: a. Gender assigment b. Genital surgery (in accordance with national regulations) c. Gonadectomy | Strong |
Do not underestimate the significant effects on psychological and psychiatric health, quality of life, personal relationships, and sexual function in individuals with DSD. | Strong |
Ensure full disclosure to patients and caregivers that the presence of a Y-chromosome in dysgenetic gonads results in a higher malignancy risk | Strong |
3.18. Congenital lower urinary tract obstruction (CLUTO)
The term congenital lower urinary tract obstruction (CLUTO) is used for a foetus, which during intrauterine US screening shows a dilatation of the upper and lower urinary tract. During pregnancy the diagnosis is usually based only on US examinations. There is a broad spectrum of conditions, that could cause an intrauterine dilatation of the urinary tract. Post-partum diagnosis comprises any anatomical and functional disorder/anomaly/malformation causing a dilatation e.g. posterior/anterior urethral valve, urethral atresia/dysplasia/stenosis prune belly syndrome, dilating reflux cloacal malformation, ureterocele, a megacystis-microcolonintestinal hypoperistalsis (MMIHS) or megacystis-megaureter syndrome [1260-1263].
Megacystis
In the first trimester, foetal megacystis is defined as a bladder with a longitudinal diameter > 7 mm, and in the 2nd and 3rd trimester as an enlarged bladder failing to empty during an extended US examination lasting at least 40 minutes. Two thirds of cases are secondary to CLUTO and the remainder are associated with genetic syndromes, developmental or chromosomal abnormalities including anorectal malformations; 14% were normal or having isolated urological abnormality (e.g. VUR, Duplex system) [1264]. A more recent systematic review showed that at least 45% of cases have oligohydramnios and 15% have chromosomal abnormalities, most of them being trisomy 13, 18 and 21. Final diagnoses were posterior urethral valve (PUV) (57%), urethral atresia/stenosis (7%), prune-belly syndrome (4%), MMIHS (1%), cloacal abnormality (0.7%) and undefined pathologies (36.5%). Termination of pregnancy rate was 50% [1265].
The prognosis of the foetus depends on the underlying pathology, the timing of diagnosis, presence of an oligo-, anhydramnios and bladder volume. Fontanella et al., developed a staging system of CLUTO. They described three groups: severe (bladder volume > 5.4 cm3 and/or oligo- or an-hydramnios, before 20 weeks), moderate (bladder volume < 5.4 cm3 and/or normal amniotic fluid at 20 weeks) and mild (normal amniotic fluid at 26 weeks) [1266]. This staging system can be used to predict perinatal mortality and post-natal estimated GFR. Another systematic review on prognosis of megacystis patients revealed an overall intrauterine spontaneous resolution of 32%, with better resolution rates in early (before 18 weeks) megacystis cases (40% vs. 12%) [1267].
3.18.1. Posterior urethral valves
3.18.1.1. Epidemiology, aetiology and pathophysiology
Posterior urethral valves (PUV) are one of the few life-threatening congenital anomalies of the urinary tract found during the neonatal period. A recent systematic review showed, that the risk for chronic kidney disease (CKD) could be up to 32% and for end-stage kidney disease (ESKD) up to 20% [1268]. Up to 17% of paediatric ESKD can be attributed to PUV [1269]. An incidence of PUV of 1 in 7,000-8,000 live-births has been estimated [1261,1270].
3.18.2. Classification systems
3.18.2.1. Urethral valve
Up until today, the original classification by Hugh Hampton Young is the most commonly used classification [1271]. Hugh Hampton Young described three categories: type I, type II and type III. However, today, only type I and type III are found to be obstructive. As type II seems to be more like a fold and not obstructive, it is no longer referred to as a valve. Hampton Young’s descriptions of type I and III are as follows:
Type I (90-95%). ‘In the most common type there is a ridge lying on the floor of the urethra, continuous with the verumontanum, which takes an anterior course and divides into two fork-like processes in the region of the bulbomembranous junction. These processes are continued as thin membranous sheets, direct upward and forward which may be attached to the urethra throughout its entire circumference. It is generally supposed that the valves have complete fusion anteriorly, leaving only an open channel at the posterior urethral wall. Yet, the fusion of the valves anteriorly may not be complete in all cases, and at this point a slight separation of the folds exists’ [1271].
Type III. There is a third type which has been found at different levels of the posterior urethra and which apparently bears no such relation to the verumontanum. This obstruction was attached to the entire circumference of the urethra, with a small opening in the centre [1261]. The transverse membrane described has been attributed to incomplete dissolution from the urogenital portion of the cloacal membrane [1272]. The embryology of the urethral valves is poorly understood. The membrane may be an abnormal insertion of the mesonephric ducts into the foetal cloaca [1273].
3.18.3. Diagnostic evaluation
An obstruction above the level of the urethra affects the whole urinary tract to varying degrees.
- The prostatic urethra is distended and the ejaculatory ducts may be dilated due to urinary reflux.
- The bladder neck is hypertrophied and rigid.
- The hypertrophied bladder occasionally has multiple diverticula.
- Nearly all valve patients have dilatation of both upper urinary tracts. This may be due to the valve itself and the high pressure in the bladder, or due to obstruction of the ureterovesical junction by the hypertrophied bladder.
- If there is secondary reflux, the affected kidney functions poorly in most cases.
During prenatal US screening, bilateral hydroureteronephrosis and a distended bladder are suspicious signs of a urethral valve. A thick-walled bladder seems to be of better prediction of a PUV than a dilated posterior urethra (‘keyhole’ sign) [1274]. However, differentiation between obstructive and non-obstructive aetiologies on prenatal US is challenging as both have a similar US appearance [1275]. In the presence of increased echogenicity of the kidney, dilatation of the urinary tract and oligohydramnion, the diagnosis of a PUV should strongly be considered. Prenatal US is adequate in most of the cases (90%) [1276]. However, in some circumstances when technical US conditions are poor, such as with oligo- or anhydramnios, large maternal body habitus, unfavourable position of the foetus or suspicion of complex foetal anomalies such as accompanying gastrointestinal system, foetal MRI may provide additional information [1276-1278].
Post-natally, a voiding cystourethrogram confirms the diagnosis of a PUV. This study is essential whenever there is a question of an infravesical obstruction, as the urethral anatomy is well-outlined during voiding. A secondary reflux is observed in at least 50% of patients with PUV [1279]. Reflux is consistently associated with renal dysplasia in patients with PUV. It is generally accepted that reflux in the renal units acts as a ‘pressure pop-off valve’, which would protect the other kidney, leading to a better prognosis [1280]. Other types of pop-off mechanisms include bladder diverticula and urinary extravasation, with or without urinary ascites [1281]. However, in the long-term, this supposed protective effect did not show a significant difference compared to other patients with PUV [1282,1283].
Nuclear renography with split renal function is important to assess kidney function (DMSA or MAG3). Creatinine, blood urea nitrogen and electrolytes should be monitored closely during the first few days. Initial management includes a multi-disciplinary team involving a paediatric nephrologist. The clinician must be aware of a noteworthy association between PUV and undescended testes and/or inguinal hernia [1284]. Undescended testes occurred in 12-17% of PUV which is consistent with a 10-fold increase [1285].
3.18.4. Management
3.18.4.1. Antenatal treatment
Today most of PUV are discovered before birth [1260-1263]. The intrauterine obstruction leads to a decreased urine output, which could result in an oligo- or anhydramnios. Amniotic fluid is necessary for normal development of the lung and its absence may lead to pulmonary hypoplasia, causing a life-threatening problem.
Kidneys start to produce urine at around tenth week of antenatal life. Many of the megacystis cases (7-15 mm) with normal karyotype spontaneously resolve before 20 weeks, whereas it is unlikely in those with a bladder length > 15mm (> 12mm before age of 18 Weeks of gestation) [1275,1286,1287]. Antenatal imaging of kidneys before 20 weeks is difficult and in rare instances imaging could be done earlier via transvaginal route [1288]. The possible spontaneous resolution chance of bladder enlargement and timing of proper kidney imaging are possibly the main obstacles on the optimum timing for prenatal intervention.
As renal dysplasia is not reversible, it is important to identify those foetuses with good renal function. A sodium level below 100 mmol/L, a chloride value of < 90 mmol/L and an osmolarity below 200 mOsm/L found in three foetal urine samples gained on three different days are associated with a better prognosis [1289]. Urine samples before 23 weeks of gestation (ß2-microglobline, sodium, chloride and calcium) may be helpful to distinguish between those who could benefit from intrauterine therapy and those in whom the outcome is most likely to be compromised [1290]. The status of amniotic fluid, the appearance of the kidneys as well as the foetal urine biochemistry could be helpful in counselling the caregivers.
Prenatal interventions aim to restore amniotic fluid volume and attenuate the risk of pulmonary hypoplasia or further renal damage [1291]. Decision for prenatal intervention can be based on a staging system that is composed of renal ultrasonographic findings, amnion amount and foetal urine biochemistry [1262]. Early intervention – before the age of 16 weeks of gestation, may be beneficial for the renal function, however making the correct diagnosis and the detection of other severe co-morbidties is extremly difficult at this time point [1292]. Later interventions are mostly of benefit for the lung development, but not for renal function.
The placing of a vesicoamniotic shunt has a complication rate of 21-59% with dislocation of the shunt being the most common one [1291]. The PLUTO-trail (randomised study) failed to show any long-term benefit on renal function by placing a visual analogue scale (VAS) [1293]. A recent meta-analysis on interventions for CLUTO reported that VAS resulted in a higher perinatal survival rate than conservative management (57.1% vs 38.8%) with no significant differences in 6-12 month survival, 2-year survival or postnatal renal function [1294]. Foetal cystoscopy with laser ablation has a high complication rate without evidence for the effectiveness of these interventions [1295]. To avoid the severe complication of the laser ablation, balloon dilation is tried [1296]. The number of patients included and designs of these studies are insufficient to give any recommendations. Parental information is very important. The natural history of CLUTO including the postnatal outcomes, with or without prenatal treatment, as well as the uncertainties and/or controversies about CLUTO diagnosis and treatment should be discussed [1291].
3.18.4.2. Postnatal treatment
Bladder drainage. If a boy is born with suspected PUV, drainage of the bladder and, if possible, an immediate VCUG is necessary. A neonate can be catheterised with a small catheter without a balloon, preferably a feeding tube. A VCUG is performed to see if the diagnosis is correct and whether the catheter is within the bladder and not in the posterior urethra. An alternative option is to place a suprapubic catheter, perform a VCUG and leave the tube until the neonate is stable enough to perform an endoscopic incision or resection of the valve.
Valve ablation. When the medical situation of the neonate has stabilised and the creatinine level decreased, the next step is to remove the intravesical obstruction. In cases where the urethra is too small to safely pass a small foetal cystoscope, a suprapubic diversion is performed until valve ablation can be performed. Small paediatric cystoscopes and resectoscopes are now available either to incise, ablate or to resect the valve at the 4-5, 7-8 or 12 o’clock position, or at all three positions, depending on the surgeon’s preference. It is important to avoid extensive electrocoagulation, as the most common complication of this procedure is stricture formation. Two studies demonstrated a lower urethral stricture rate using the cold knife compared to diathermy [1297,1298]. Within the three months following initial treatment, effectiveness of the treatment should be demonstrated either by clinical improvement (US and renal function), control VCUG or a re-look cystoscopy, depending on the clinical course [1299-1301] .
Vesicostomy. If the child is too small and/or too ill to undergo endoscopic surgery, a suprapubic diversion is performed to drain the bladder temporarily. If initially a suprapubic tube has been inserted, this can be left in place for six to twelve weeks. Otherwise, a cutaneous vesicostomy provides an improvement or stabilisation of the UUT in up tp 90% of cases [1302,1303]. Although there has been concern that a vesicostomy could decrease bladder compliance or capacity, so far there are no valid data to support these expectations [1304,1305]. Moreover, it was shown in PUV patients with stage 3 CKD that adding vesicostomy to valve ablation no long-term benefit was noted from diversion in the ultimate incidence of ESKD [1306].
High diversion. If bladder drainage is insufficient to drain the UUT, high urinary diversion should be considered. Diversion may be suitable if there are recurrent infections of the upper tract, no improvement in renal function and/or an increase in upper tract dilatation, despite adequate bladder drainage. The choice of urinary diversion depends on the surgeon’s preference for high-loop ureterostomy, ring ureterostomy, end ureterostomy or pyelostomy, with each technique having advantages and disadvantages [1307-1310]. Diversion can delay progression to end stage renal failure [1306]. Reconstructive surgery should be delayed until the UUT has improved as much as can be expected.
Reflux is very common in PUV patients (up to 72%) and it is described bilaterally in up to 32% [1311]. During the first months of life, antibiotic prophylaxis may be given especially in those with high-grade reflux [959] and in those with a phimosis, circumcision can be discussed in order to reduce the risk of UTIs [1312]. However, there are no randomised studies to support this for patients with PUV. Early administration of oxybutynin may improve bladder function as shown in one study with eighteen patients [1313]. High-grade reflux is associated with a poor functioning kidney and is considered a poor prognostic factor [1314,1315]. However, early removal of the renal unit seems to be unnecessary, as long as it causes no problems. Moreover, in the long term it may be necessary to augment the bladder and in this case the ureter may be used [1316]. Deterioration of renal function without a fixed obstruction and higher urine output (polyuria) may lead to an overdistension of the bladder during the night. Drainage of the bladder during the night by a catheter may be beneficial for the hydronephrosis as well as for renal function [1317,1318]. Patients with high daytime PVR urine may benefit from CIC [1319,1320]. In those who do not want or are not able to perform a CIC via urethra, the placement of a Mitrofanoff is a good alternative [1321].
3.18.5. Follow-up
Several prognostic factors have been described. Different serum nadir creatinine levels are given in the literature (0.85 mg/dl-1.2 mg/dl [μmol/L]) [1322-1325]. Renal parenchyma quantity (total renal parenchymal area) and quality (corticomedullary differentiation and renal echogenicity) on initial postnatal US also have prognostic value [1326].
Life-long monitoring of these patients is mandatory, as bladder dysfunction (‘valve bladder’) is not uncommon and the delay in day- and night-time continence is a major problem [1327,1328]. The literature demonstrates that urodynamic studies play an important role in the management of patients with valve bladder especially in those with suspicion of bladder dysfunction [1329,1330]. Poor bladder sensation and compliance, detrusor instability and polyuria (especially at night) and their combination are responsible for bladder dysfunction. In those with bladder instability, anticholinergic therapy can improve bladder function. However, there is a low risk of reversible myogenic failure (3/37 patients in one study) [1331,1332]. In patients with poor bladder emptying, α-blocker can be used to reduce the PVR urine, as demonstrated in one study with 42 patients using terazosin (mean PVR was reduced from 16 to 2 mL) [1333]; in another study tamsulosin was effective [1334]. Concerning bladder neck incision, there is no Panel consensus concerning indication and efficacy. High creatinine nadir (> 1 mg/dL) and severe bladder dysfunction are risk factors for renal replacement therapy [1335,1336]. Renal transplantation in these patients can be performed safely and effectively [1337,1338]. Deterioration of the graft function is mainly related to LUTD [1337]. Therefore, it is essential to have and keep a good reservoir function. An assessment and treatment algorithm is provided in Figure 12.
There are only few reports on sexual function and fertility in patients with PUV demonstrating some impairment especially in those who are on dialysis [1339,1340]. In a review the majority have good erectile function (74- 94%) and a fertility comparable to the normal population [1341]. However, a negative influence of the individual patient’s fertility has to be taken into account, as these patients have a higher risk for bilateral cryptorchidism, recurrent epididymitis and ESRD [1341].
Figure 12: An algorithm on the assessment, management and follow-up of newborns with possible PUV CIC = clean intermittent catheterisation; OAB = overactive bladder; PUV = posterior urethral valve;RF = renal function; UT = urinary tract; UUT = upper urinary tract; VCUG = voiding cystourethrogram.
CIC = clean intermittent catheterisation; OAB = overactive bladder; PUV = posterior urethral valve;RF = renal function; UT = urinary tract; UUT = upper urinary tract; VCUG = voiding cystourethrogram.
3.18.6. Summary
Posterior urethral valves are one of the few life-threatening congenital anomalies of the urinary tract found during the neonatal period and despite optimal treatment result in renal insufficiency in nearly one-third of cases. Bilateral hydroureteronephrosis and a distended bladder are suspicious signs of a PUV in neonates. A VCUG confirms a PUV diagnosis. Nuclear renography with split renal function is important to assess kidney function and serum creatinine nadir above 80 μmol/L is correlated with a poor prognosis. Today, antenatal therapy is becoming more and more popular. Identification of those with an obstructive uropathy and definiton of those who would benefit from early antenatal intervention are the major challenges. Postnatal treatment includes bladder drainage, either transurethral or suprapubic and if the child is stable enough, endoscopic incision of the valve is performed. If a child is too small and/or too ill to undergo endoscopic surgery, a vesicostomy is an option for bladder drainage. If bladder drainage is insufficient to drain the UUT, high urinary diversion should be considered.
In all patients life-long monitoring is mandatory, as bladder dysfunction is quite common and may cause progressive upper tract deterioration, if not managed properly. In the long-term between 10 and 47% of patients may develop end-stage renal failure. Renal transplantation in these patients can be performed safely and effectively.
- Anterior urethral valve (AUV)
Anterior urethral valve is a semilunar or iris-like band of tissue on ventral aspect of urethra. It can be isolated, in association with or confused with urethral diverticulum. The aetiology of isolated AUV is speculated to be secondary to congenital urethral obstruction, malunion of glanular and penile urethra, congenital cystic dilatation of peri-urethral glands or ruptured distal lip of a syringocele [1342]. Anterior urethral valve occurs less frequently than PUV. It can be present in the bulbous urethra, the penoscrotal junction and penile urethra. Patients may present with poor urinary stream, penile ballooning, UTI or haematuria. Anterior urethral valves have been classified by Firlit et al., depending on the presence of diverticulum and the dilatation of urethra and upper tract [1343]. The diagnosis is based on VCUG with possible findings of dilated or elongated posterior urethra, a dilatation of the anterior urethra, a thickened trabeculated bladder, a hypertrophied bladder neck, VUR, and urethral diverticula. In doubtful cases, retrograde urethrography may be helpful showing linear filling defect along the ventral wall, or it may show a dilated urethra ending in a smooth bulge or an abrupt change in the caliber of the dilated urethra on VCUG [1344]. Treatment is performed mainly by endoscopic valve ablation. In selected patients, a temporary diversion may be considered until the child is big enough for endoscopy to be possible. Open surgery is reserved in patients with very large diverticulum and defective spongiosum. Renal failure may develop in 22% and the risk is highest in patients with pre-treatment azotaemia, VUR and UTI [1345].
- Anterior urethral diverticulum (AUD):
Common postnatal presenting features of AUD are compressible ventral penile swelling, urinary dribble postmicturition, voiding difficulty, poor stream, and recurrent UTIs [1346-1348]. Diagnosis is made by VCUG with or without a retrograde urethrogram. In small AUD, endoscopic cutting or deroofing of distal lip of the diverticulum can be used as a treatment modality. Larger diverticulum requires excision of the diverticulum with a two layered urethroplasty; or marsupialisation with staged urethroplasty. In cases of urosepsis and obstructive uropathy, a suprapubic catheter may be placed. Once the infant’s condition improves, temporary urinary diversion with vesicostomy or proximal cutaneous urethrostomy can be performed before definitive surgical management [1349,1350]. The diverticulum is associated with a distal lip-like tissue which may be confused with a valve. Anatomically, AUV have normal corpus spongiosum development whereas AUD have incomplete spongious tissue formation [1349].
- Syringocele
Cowper glands are two bulbourethral glands located within the urogenital diaphragm and secrete pre-ejaculatory mucus on both sides through the external sphincter into the urethra 1-2 cm distal to the sphincter. Syringocele is the cystic dilatation of these glands. The aetiology can be congenital (retention cyst of the intraurethral portion of the duct) or acquired (trauma or infection). It has been classified as simple, imperforate, perforate and ruptured [1351]. A simpler grouping is suggested to merge simple, perforate and ruptured into “open syringocele” and imperforate to “closed syringocele”. Closed syringoceles cause obstructive symptoms and open ones act as a diverticula and cause post-voiding drippling and sometimes obstruction due to orientation of one membrane into urethra [1352]. However, it is better to simply categorise into two groups as obstructing and non-obstructing in terms of understanding pathophysiology and management [1353]. Depending on the syringocele type, patients present with post-void dribbling, urethral discharge, UTI, perineal pain, haematuria, obstructive voiding symptoms, dysuria and retention. Diagnosis is based on antegrade and/ or retrograde urethrogram which shows a cystic defect distal to prostate. If the VUC/RGU are inconclusive, US and/or MRI may be used if open reconstruction is being planned. Endoscopic deroofing of the cyst in both obstructing and non-obstructing syringoceles is an effective method of marsupialisation [1354]. In cases where endoscopic approach is not feasible open correction may be considered.
- Cobb’s collar
Cobb’s collar is a congenital membranous stricture of the bulbar urethra. It is different from congenital obstructive posterior urethral membrane (COPUM) and is independent of the verumontanum and external sphincter and may represent a persistence of part of the urogenital membrane [1355]. Voiding cystourethrogram shows narrowing in the proximal bulbar urethra with folds extending proximally, a dilated posterior urethra, prominent bladder neck and other findings of infravesical obstruction. Treatment is an endoscopic incision; using cold-knife showed lower recurrence rates than electrocautery [1356].
- Urethral atresia/hypoplasia
Male urethral atresia is a congenital, complete obstruction of the urethra caused by a membrane that is usually located at the distal end of the prostatic urethra. The urethra distal to this point is usually hypoplastic, presumably from lack of foetal voiding [1357]. Urethral atresia is associated with bladder distention, VUR, hydronephrosis and renal dysplasia [1358]. Most cases reported have the phenotypic characteristics of the prune belly syndrome. Antenatal intervention may be beneficial in terms of foetal survival [1359]. Although progressive augmentation by dilating the urethra anterior (PADUA) procedure was described as a treatment modality, the majority of cases requires some form of supravesical diversion [1357,1358].
- Posterior Urethral Polyps:
Although, posterior urethral polyps (PUP) does not cause ANH, it could cause obstruction later in life. Posterior Urethral Polyps is a polypoid, pedunculated, fibroepithelial lesion arising in posterior urethra proximal to the verumontanum. It lies on the floor of the urethra with its tip reaching into the bladder neck and obstruction occurs because of distal displacement of polyp during urination [1360]. Patients complain of dysuria, haematuria and obstructive symptoms such as poor urinary stream and intermittent retention episodes. Diagnosis can be suspected by VCUG and/or US but is confirmed during cystourethroscopy. Treatment is usually an endoscopic resection of the polyp. The course of the disease is benign and no recurrences were reported in the literature [1361,1362].
3.18.7. Summary of evidence and recommendations for the management of posterior urethral valves
Summary of evidence | LE |
Posterior urethral valves are one of the few life-threatening congenital anomalies of the urinary tract found during the neonatal period. | 1b |
Antenatal therapy could be discussed based on ultrasound findings, fetal urine biochemistry amount of amnion fluid and chromosomal status. | 4 |
Despite optimal treatment nearly one-third of the patients end up in renal insufficiency. | 2b |
Bilateral hydroureteronephrosis and a distended bladder are suspicious signs on US; a VCUG confirms the diagnosis. | 2b |
Serum creatinine nadir above 85 μmol/L is correlated with a poor prognosis. | 2a |
In the long-term up to 20% of patients develop end-stage renal failure due to primary dysplasia and/or further deterioration because of bladder dysfunction. Renal transplantation in these patients is safe and effective, if the bladder function is normalised. | 2a |
Recommendations | LE | Strength rating |
Diagnose posterior urethral valves (PUV) initially by ultrasound but a voiding cystourethrogram (VCUG) is required to confirm the diagnosis. | 3 | Strong |
Assess split renal function by dimercaptosuccinic acid scan or mercaptoacetyltriglycine (MAG3) clearance. Use serum creatinine as a prognostic marker. | - | Strong |
Vesico-amniotic shunt antenatally is not recommended to improve renal outcome. | 1b | Weak |
Offer endoscopic valve ablation after bladder drainage and stabilisation of the child. | 3 | Strong |
Offer suprapubic diversion for bladder drainage if the child is too small for valve ablation. | - | Strong |
Offer a high urinary diversion if bladder drainage is insufficient to drain the upper urinary tract and the child remains unstable. | - | Strong |
Monitor bladder and renal function life-long, in all patients. | 3 | Strong |
3.19. Rare Conditions in Childhood
3.19.1. Urachal remnants
3.19.1.1. Introduction
The urachus is an embryonic structure arising as a result of the separation of the allantois from the ventral cloaca. The allantois appears on day sixteen as a tiny, fingerlike outpouching from the caudal wall of the yolk sac, which is contiguous with the ventral cloaca at one end and the umbilicus at the other. The ventral portion of the cloaca develops into the bladder after cloacal division by the urogenital septum. Thus, the bladder initially extends all the way to the umbilicus [1363]. With progressive foetal development, as the bladder descends into the pelvis, the attachment between the umbilicus and the urachus becomes looser and the apical portion progressively narrows to a small, epithelialised, fibromuscular strand by the fourth or fifth month of gestation. The urachus then obliterates completely by birth, forming the median umbilical ligament [1364-1366].
The urachus varies from 3 to 10 cm in length and from 8 to 10 mm in diameter. It is a three-layered tubular structure, the innermost layer being lined with transitional epithelium, the middle layer composed of connective tissue, and the outermost muscular layer in continuity with the detrusor muscle [1367].
Urachal remnants (URs) originate from failure of the obliteration of the allantois, resulting in a urachal anomaly such as (1) urachal sinus, (2) urachal cyst, (3) vesico-urachal diverticulum, and (4) patent urachus [1364,1365,1368]. Most often the urachal anomaly is asymptomatic, but it occasionally may become infected, may cause urinary symptoms, or develop a urachal carcinoma in later life [1367,1369].
3.19.1.2. Epidemiology
Reports of occurrence rates in the literature vary broadly from a very rare disease in the older literature to a fairly common problem. Robert et al. found that URs were present in 61.7% of patients younger than 16 years [1370]. They also noted that the frequency of URs decreased with increasing age. This supports a physiological regression of URs with age. Stopak et al. attributed this upsurge to increased awareness among community paediatricians and improvements in US that made visualisation of urachal remnants easier [1371].
Clinical studies and paediatric autopsy studies in the past have shown a much lower incidence. Rubin found an incidence of 1 in 7,610 cases of patent urachus and 1 in 5,000 cases of urachal cysts [1372]. Nix et al., noted three anomalies out of 1,168,760 hospital admissions, and Blichert-Toft et al., reported five UR cases out of 40,000 patients [1373,1374]. The incidence rate in males is a little higher than in females [1375,1376].
The range of the various URs reported in the literature is 10% to 48% for patent urachus, 31% to 43% for urachal cyst, 18% to 43% for urachal sinus and 3% to 4% for urachal diverticulum [1377,1378].
3.19.1.3. Symptoms
A patent urachus causes continuous or intermittent urine leakage from the umbilicus causing umbilical granulation and erythema in infants [1377]. A urachal cyst is usually diagnosed (1) incidentally, or (2) when it becomes infected causing abdominal pain and discharge of pus from the umbilicus or recurrent UTIs when it drains into the bladder.
The most common symptom is umbilical granulation, discharge and erythema in infants and abdominal pain in older children [1377].
Other symptoms of infected urachal anomalies can vary from high fever, abdominal pain, urinary tract infections, LUTS and/or an abdominal mass [1378-1382]. A urachal diverticulum is often asymptomatic and is usually found incidentally during investigations for other problems. An alternating sinus can empty either into the bladder or the umbilicus and this characteristic is responsible for various presentations [1383]. Infection has been reported as the most common complication in urachal anomalies [1384]. Severe infection may develop into peritonitis and sepsis. Cultures from umbilical discharge usually show Staphylococcus, Streptococcus and E. Coli [1385].
- Other congenital anomalies:
Ashley found a simultaneous anomaly in 17 of 46 children, of which VUR was the most common anomaly (6 patients) [1386]. Other investigators reported associated anomalies in cases of persistent URs including meatal stenosis, hypospadias, umbilical and inguinal hernias, cryptorchidism, anal atresia, omphalocele, ureteropelvic obstruction and most frequently, VUR [1376,1387-1389].
3.19.1.4. Diagnosis
In the majority of cases with complaints of a UR, a careful history and physical examination will confirm the suspicion of a UR. In many patients this can be confirmed by US studies [1370]. An MRI or CT scan may be necessary in a minority of children [1381]. Because of the association with other congenital abnormalities, other studies such as a VCUG or cystoscopy may be undertaken as well. In general, the VCUG is only undertaken when the child also presents with UTI or when the US shows signs of upper tract abnormalities. For the diagnosis per se it is not necessary [1390]; however, a VCUG may be useful for defining the type of urachal anomaly and evaluating a population that may be at higher risk for VUR.
3.19.1.5. Treatment
If a UR is symptomatic, the standard approach has been surgical removal. In most cases it should be done as an elective procedure, following appropriate treatment of active inflammation, and infection is possible. Pre-operative IV-dosage of antibiotic like Cefazolin is generally sufficient. A Pfannenstiel, periumbilical or infraumbilical midline incision can all be used for the open surgical approach [1380,1391]. Even in symptomatic infants a more conservative approach is possible as well, especially in children less than six months old. Observation and treatment with antibiotics if necessary and radiographic monitoring are a safe approach [1377,1392,1393]. Dethlefs et al., reported a 90% successful outcome [1379], while Naiditch et al. reported that 44 of 78 symptomatic patients resolved under observation [1382]. More recently the laparoscopic approach has been advocated, and shown to be safe [1393-1395]. Surgery is not without risk. The rate of complications following surgical removal varies from 0 to 20%: usually wound infections [1371,1379-1382,1391]. Considering the probable additional risk of anaesthesia in very young children any surgical procedure needs to be assessed carefully [1396,1397].
3.19.1.6. Pathology of removed remnants
Removed specimens may show inflammation or a cystic structure [1379]. Patients presenting without symptoms are as likely to have epithelial elements in the UR as those presenting with symptoms [1381].
3.19.1.7. Urachal cancer
Urachal anomalies are thought to be associated with an increased risk of bladder adenocarcinoma in adults, and urachal adenocarcinoma has an estimated incidence of 0.18 per 100,000 individuals yearly [1398]. These cases account for 0.1 to 0.3% of all bladder malignancies and 20 to 39% of bladder adenocarcinomas [1399]. Urachal adenocarcinoma (UrC) is very rare, especially when one considers that up to 62% of children under 16 years of age may have a UR [1370,1400]. A study by Copp et al. found no association between the presence of UR symptoms and the presence or absence of epithelial tissue in pathology specimens, leading them to conclude that UR symptoms have poor predictive value for malignancy potential in these remnants [1376].
Gleason et al., found that 5,721 URs would need to be excised to prevent a single case of urachaladenocarcinoma out of the nearly 65,000 patients reviewed [1398]. Assuming that epithelium is required in the development of urachal adenocarcinoma, the extrapolated Number Needed to Treat (NNT) would be more than 8,000, as nearly 30% of urachal anomalies are void of an epithelial component. Less than 5% of urachal cancers have a non-epithelial origin such as sarcoma [1401]. The presenting symptoms in adults are different from those in children: in a study of 130 adult patients, Ashley et al., found that 49% presented with haematuria and 27% with pain. In 51% a urachal carcinoma was diagnosed: adenocarcinoma, with 58% high grade cancer. In addition, 20% had metastases at diagnosis, the overall 5-year cancer specific survival rate in the UrC cohort was 49% [1402]. Stasis of urine and crystallisation promotors such as mucus or desquamated epithelium in the UR are most likely the cause for malignant degeneration as well as stone formation in the adult patient. At present no long-term follow-up on untreated UR in children is available and there is no evidence that urachal anomalies in children increase the likelihood of future malignancy [1377,1403].
3.19.1.8. Conclusion
Urachal remnants appear to be more common than previously reported. During the first 6-12 months of life spontaneous resolution is common. Excision of symptomatic urachal anomalies is an effective and safe means of treatment, with minimal morbidity. However, most patients with simple and asymptomatic lesions do not appear to benefit from excision, as the risk of malignancy later in life is vanishingly remote. Early intervention (< 6 months of age) should be reserved for patients with persistent documented urine draining from the urachus or a documented abscess. Incidental (US) UR management remains a challenge and should be done with patient and family involvement to make the most informed decision. While surgical intervention has minimal risk and morbidity, it is performed unnecessarily in a large proportion of asymptomatic patients due to the unnecessary removal of non-epithelial containing urachal anomalies and the inability to predict which anomalies will undergo malignant transformation [1404].
3.19.1.9. Recommendation for management of urachal remnants
Recommendations | Strength rating |
Urachal remnants (URs) with no epithelial tissue carry little risk of malignant transformation. | Strong |
Asymptomatic and non-specific atretic urachal remnants can safely be managed non-operatively. | Strong |
Urachal remnants incidentally identified during diagnostic imaging for non-specific symptoms should also be observed non-operatively since they tend to resolve spontaneously. | Strong |
A small UR, especially at birth, may be viewed as physiological. | Strong |
Urachal remnants in patients younger than six months are likely to resolve with non-operative management. | Strong |
Follow-up is necessary only when symptomatic for six to twelve months. | Strong |
Surgical excision of URs solely as a preventive measure against later malignancy appears to have minimal support in the literature. | Strong |
Only symptomatic URs should be safely removed by open or laparoscopic approach. | Strong |
A voiding cystourethogram is only recommended when presenting with febrile urinary tract infection. |
3.19.2. Papillary tumours of the bladder in children and adolescents (Papillary urothelial neoplasm of low malignant potential or transitional cell carcinoma)
3.19.2.1. Incidence
Papillary tumours of the bladder in children and adolescents are extremely rare and are different from papillary tumours in adults. A “grape-like” papillary tumour in young children will be more likely a rhabdomyosarcoma of the bladder, which are not the focus of this guideline. A papillary tumour in older children or adolescents will be more likely be a papillary urothelial neoplasm of low malignant potential (PUNLMP) [1405]. Children with risk factors, such as previous bladder surgery and immunosuppressive medication can also develop a nephrogenic adenoma of the bladder, also presenting as a papillary tumour of the bladder.
3.19.2.2. Differences and similarities of papillary tumours of the bladder in children and adults
Gender
The overall the risk of a papillary tumour in the bladder in paediatric and young adult patients is approximately double in males compared to females [1406].
3.19.2.3. Risk factors
The majority of these patients have no identifiable risk factors.
3.19.2.4. Presentation
The most common symptom at presentation is haematuria; other less common symptoms include abdominal pain, storage LUTS including frequency, dysuria and at times obstructive symptoms [1406].
3.19.2.5. Investigations and treatment
Ultrasound of the genitourinary tract is the first investigation of choice. It is an excellent screening tool and can often accurately diagnose the nature and location of lesion. In children and adolescents, a bladder US of the full bladder is more sensitive compared with adults due to reduced abdominal fat and thinner muscle layer [1407]. In the event of a need to differentiate the renal or bladder origin of the haematuria, a red blood cell morphology will reveal isomorphic blood cells, differentiating a bladder origin. Urine cytology can be performed, however it has very limited value likely due to the low-grade nature of these tumours in children. Cystoscopy should be reserved if a bladder tumour is suspected on imaging for simultaneous diagnosis and treatment, transurethral resection of the tumour. In children, cystoscopy requires general anaesthesia [1408].
3.19.2.6. Histology
All the lesions in the children and adolescent age-group are identified as papillary and over 85% are solitary [1407]. Papillary bladder tumours in patients younger than twenty years of age have low-grade non-invasive disease (WHO classification) [1409]. These findings let pathologists conclude that in children and adolescents, a papillary bladder tumour can be classified as Papillary Urothelial Neoplasm of Low Malignant Potential (PUNLMP). PUNLMP has minimal or no cytological atypia and it differs from low grade transitional cell carcinoma (TCC) which has cytologic atypia, hyperchromatic nuclei and scattered mitosis [1410].
3.19.2.7. Additional treatment
Mitomycin C and Bacillus Calmette-Guérin have both been used in children but there is no evidence of their efficacy due to the rarity of TCC, and especially of high grade TCC [1406]. Hence, as per current evidence, there is no place for instillations in children.
3.19.2.8. Prognosis, recurrence and surveillance
The prognosis of papillary tumours of the bladder in children is overall good. The recurrence rate in children and adolescents varies from 8 to 15% [1405-1407]. Mean time to recurrence can vary from 11 to 29 months depending on the study, with recurrences occurring up to 90 months from diagnosis; though 64% occur in the first year [1406]. In certain cases, recurrences can be fairly aggressive [1407].
Strategies are based on the guidelines and protocols of papillary tumours of the bladder in adults. It is advised to follow-up children and adolescents with a history of a PUNLMP initially with a short interval of three to six months in the first year, and thereafter at least yearly with urinanalysis for haematuria and an US of the full bladder. In the event of sudden gross haematuria, the evaluation must be performed immediately. If the tumour was completely resected at primary surgery, standard follow-up cystoscopy is not necessary and may be reserved for children or adolescents with a high recurrence risk or suspected recurrence on bladder US [1407]. The exact duration of follow-up is unknown but this Panel recommends follow-up for at least five years.
Inflammatory myofibroblastic tumours of the bladder (IMTB) are rare with nearly 200 cases reported in the literature [1411,1412]. Around 25% occur in children with a median age at diagnosis of 7.5 years and a median tumour size of 5.5 cm. Boys and girls are equally affected [1413]. Usually these tumours are benign, with only very few reported malignant cases [1414]. Treatment is mostly surgical with transurethral resection, but local resection, or partial cystectomy maybe needed in selected cases [1413,1415]. Additionally, a conservative approach is reported [1416]. Histological examination is required to exclude other malignant tumours such as a rhabdomyosarcoma. In children, no recurrence has been reported so far. However, due to their malignant potential and few recurrences in adults, the same follow-up as for papillary bladder tumours is recommended.
Eosinophilic cystitis
Though well described in adults, this inflammatory condition is rare in the paediatric population with less than 100 cases reported in the literature to date [1417]. Its a etiology remains unknown, but is thought to be incited by IgE mediated attraction of eosinophils to bladder wall followed by mast cell degranulation. It has been linked to medications, specifically antibiotics such as penicillin, chemotherapeutic agents e.g. cyclophosphamide and mitomycin, and chronic bladder catheterisation [1418,1419]. In children, as opposed to adults, males are more frequently afflicted with seven years being the mean age of presentation, however the condition can be seen throughout childhood even in LUTS [1417,1420].
Irritative bladder symptoms such as dysuria, frequency, urgency and incontinence are the most frequent and can mimic UTI [1421]. Other symptoms include haematuria, suprapubic tenderness and systemic symptoms. Obstructive manifestations due to mass formation in the bladder wall can result in ureteral obstruction leading to hydro-ureteronephrosis, suprapubic mass in infants in addition to voiding dysfunction [1417,1420,1422].
Although associated with allergy only about a third of reported cases had a history of other allergic conditions whereas half had significant eosinophilia or eosinophiluria. Diagnosis is often delayed as symptoms of eosinophilic cystitis (EC) mimic other more common conditions such as UTI and LUTS and most patients will ultimately have undergone imaging studies such as ultrasound, VCUG, CT and MRI, which although not specially diagnostic for the condition, may show bladder wall thickening or even mass formation, with rhabdomyosarcoma constituting an important differential diagnosis. A high index of suspicion for the diagnosis should therefore be maintained when dealing with protracted urinary symptoms not responsive to conventional intervention. Definitive diagnosis can only be attained on tissue biopsy obtained by cystoscopy. Histologically, eosinophilic infiltration of lamina propria and muscularis are seen in acute phases with > 25 eosinophils per high power field considered to be significant [1417,1420,1422]. Management is not standardised; removal of any possible allergens is the obvious first step and there are reports of self-limiting course of the disease. However, empirical treatment with corticosteroids, antibiotics, anticholinergics, and antihistamines, in addition to cyclosporine A have been utilised and lead to resolution of symptoms in most cases. Partial cystectomy has been performed in circumscribed lesions that do not disappear spontaneously. No standard follow-up recommendations exist however surveillance is justified as recurrence has been reported in about a third of patients [1417,1420].
Nephrogenic adenoma
Nephrogenic adenomas (NA) in children are rare benign lesions that usually occur in the setting of previous surgery or chronic irritation of urinary tract [1423]. These benign proliferative lesions are most commonly found in the bladder. There is a significant predominance of girls compared to boys (5:1). The exact pathogenesis is unknown. It is proposed to be a metaplastic process of native urothelium in response to chronic injury. Recent evidence suggest that they can be derived from renal tubular cells that shed, migrate, reimplant and proliferate within urothelial mucosa [1424]. Though they are known to occur concurrently with bladder cancer, there are no de novo cases of bladder cancer diagnosed after nephrogenic adenoma. Previous history of bladder surgery such as bladder augmentation or presence of chronic inflammation or irritation is important [1425]. Lesions tend to develop at sites prone to chronic catheterisation injury. Other risk factors include trauma, immunosuppression and radiation. They present with haematuria and storage LUTS with a papillary/ polypoid mass on cystoscopy. The recurrence rate is as high as 80% over 4 years [1423]. The final diagnosis is established by cystoscopy and histopathological review of biopsy specimen. Treatment is excision either by transurethral resection which often requires reresections, partial cystectomy or open excision. Again no standard follow-up recommendations exist however regular follow-up with cystoscopy has been advocated especially for patients with augmented bladders as recurrence seem particularly high in this subgroup [1425].
3.19.2.9. Summary of evidence and recommendations for papillary tumours of the bladder in children
Summary of evidence | LE |
Majority of paediatric patients have no identifiable risk factors for bladder tumours. | 3 |
There is no evidence on intravesical therapy for bladder tumours in children and adolescents. | 4 |
Prognosis of papillary tumours of the bladder in children is good overall. | 3 |
Inflammatory myofibroblastic bladder tumours are usually benign. | 3 |
Paediatric EC cases are in a third of cases associated with a history of allergic conditions and in 50% with significant eosinophilia or eosinophiluria. | 4 |
Paediatric EC patients usually present with irritative and or obstructive urinary symptoms which can mimic UTI or LUTS thereby leading to delayed diagnosis. | 4 |
In paediatric EC definitive diagnosis can only be attained on tissue biopsy obtained by cystoscopy. | 4 |
In EC treatment with corticosteroids, antibiotics, anticholinergics, and antihistamines, in addition to cyclosporine A have been utilised and lead to resolution of symptoms in most cases. | 4 |
No standard follow-up recommendations exist however surveillance is justified as recurrence has been reported in about a third of patients. | 4 |
NA in children are rare benign lesions that usually occur in the setting of previous surgery or chronic irritation of urinary tract and mainly occurring in the bladder. | 4 |
NA usually presents with haematuria and or storage LUTS and with a papillary/polypoid mass on seen on cystoscopy. | 4 |
NA diagnosis is established by cystoscopy and histopathological review of biopsy specimen. | 4 |
NA treatment is excision either by transurethral resection which often requires reresections, partial cystectomy or open excision. | 4 |
NA recurrence rate is high thereby justifying regular follow-up. | 4 |
Recommendations | LE | Strength rating |
Ultrasound is the first investigation of choice for the diagnosis of paediatric bladder tumours. | 3 | Strong |
Cystoscopy should be reserved if a bladder tumour is suspected on imaging for diagnosis and treatment. | 3 | Strong |
After histological confirmation, inflammatory myofibroblastic bladder tumours should be resected locally. | 4 | Weak |
Follow-up should be every three to six months in the first year, and thereafter at least. | 4 | Strong |
annually with urinanalysis and an ultrasound for at least five years. | 4 | Strong |
Have a high index of suspicion of eosinophilic cystitis (EC) in protracted urinary tract symptoms unresponsive to regular treatment. | 4 | Weak |
Remove any possible allergens as the obvious first step in managing EC. | 4 | Strong |
Eosinophilic cystitis can be managed medically with corticosteroids, antibiotics, anticholinergics, and antihistamines, in addition to cyclosporine A. | 4 | Weak |
Manage nephrogenic adenoma (NA) by resection either transuretherally or by open excision. | 4 | Strong |
Regular endoscopic follow-up especially for augmented patients with NA is justified. | 4 | Weak |
3.19.3. Penile rare conditions
Paediatric lesions of the penis are uncommon but an important part of the paediatric urological practice. The most common of these lesions are cystic penile lesions followed by vascular malformations and neurogenic lesions [1426]. Soft tissue tumours of the male external genitalia are uncommon, but have been described in the paediatric age group and can be malignant [1427].
3.19.3.1. Cystic lesions
- Epidermal inclusion cysts are the most common genital cystic lesion and can occur anywhere on the body in both men and women; in the penis it occurs most commonly over the penile shaft varying from 0.1 to 1 cm in diameter. Their epithelium is lined and filled with keratin. It is a painless swelling and can present in the age group with a history of circumcision. Treatment by total surgical excision is mainly indicated for cosmetic or symptomatic (e.g. infection) reasons and should be performed without rupturing the cyst to avoid recurrence [1428].
- Mucoid cyst of the penis is synonymous with parameatal cyst or genitoperineal cyst of median raphe; they are midline developmental cysts arising from ectopic urethral mucosa filled with mucoid material. They present since birth but are usually detected during adolescence or later. They are usually asymptomatic developing over penile ventral surface around glans and require surgical removal for either cosmetic, functional or symptomatic reasons [1429].
- Median raphe cysts arise from incomplete closure of genital fold during embryogenesis; they are commonly diagnosed in the first decade of life but can present later as they tend to be asymptomatic [1430]. They are either unilocular or multilocular fluid containing cysts, with a mean size of 0.8 cm but cysts larger than 2 cm have also been reported [1431]. Cysts are centred in dermis, with no connection to urethra or epidermis. Histopathologically, there are 4 types: urethral (urothelium-like epithelium, account for 55% cases), epidermoid, glandular and mixed. They can be treated conservatively and can resolve spontaneously or persist. Cyst aspiration is associated with high risk of recurrence and surgical excision is the treatment of choice. Though most penile cysts are asymptomatic, they may get infected resulting in pain and tenderness. They can also present with ulceration, rupture and urinary obstruction if they are close to the urethral meatus. This along with cosmetic issues means that most caregivers and patients opt for surgical excision.
- Smegmal cysts or smegmal pearls can be a differential for the cysts above; they are a benign collection of smegma in the sub-preputial space in uncircumcised boys with anticipated spontaneous resolution [1432].
- Dermoid cyst are congenital, asymptomatic, firm, solitary, subcutaneous cystic lesions occurring commonly in the region of the corona involving the foreskin. Histopathologically they contain sweat and sebaceous glands with elements of hair and squamous epithelium. Pilosebaceous cysts have been described on the glans; they are benign and usually diagnosed after excision.
3.19.3.2. Vascular malformations
A broad classification of penile vascular lesions into haemangiomas and vascular malformations was proposed by Ramos in 1999 [1433]. Haemangiomas develop rapidly at birth and involute slowly; they also include pyogenic granulomas which are benign outgrowths of cutaneous capillary vessels formed usually from chronic irritation [1426]. The growth cycle of infantile haemangiomas is divided into early and late proliferative stages, followed by a slow involution phase, completing growth by nine months of age [1434]. Propranolol is currently first line treatment for infantile haemangiomas, the exact mechanism of action is unknown but can include inhibition of angiogenesis, vasoconstriction among others. The dose is in the range of 1.5-2.5 mg/kg, which needs to be continued for 12 to 18 months and then tapered through active or passive weaning to reduce risk of rebound growth [1434]. Other factors leading to rebound growth after propranolol treatment include deep haemangiomas, which occur in about 38% patients despite propranolol therapy, requiring local therapy such as topical timolol, pulsed dye laser or intralesional steroids. After twelve months, the median improvement with treatment is reported as 81% (range 70-90%) based on VAS scores of serial patient photographs.
Vascular malformations are congenital lesions of capillary, lymphatic and venous (or slow-flow) or arterial/arteriovenous (fast-flow) origin that enlarge slowly as the patient grows. These include glomus tumours, which are primarily congenital arteriovenous shunts that develop from thermo-regulatory glomus bodies (fastflow vascular malformations). Glomus tumours of the penis can arise on the glans penis, corpora of the penis and as periurethral masses, sometimes accompanied by glomus tumours of fingers and feet [1435]. These are usually asymptomatic at presentation or may have symptoms such as priapism, palpitation and perineal pain. Glomus tumours are benign despite exhibiting high grade nuclear polymorphism. Vascular malformations are usually benign and treated either with laser, sclerotherapy or surgical excision. However, glomus tumours specifically need surgical treatment and follow-up due to the risk of recurrence from incomplete excision [1436].
3.19.3.3. Neurogenic lesions
Penile neurofibroma is an extremely rare lesion arising from perineural and Schwann cells, and occurs usually with evidence of systemic neurofibromatosis or von Recklinghausen syndrome [1437]. They are treated successfully with complete excision [1426]. Rare cases of malignant schwanomas on the penis presumably secondary to malignant transformation of benign neurofibromas have been reported in boys with a strong family history of neurofibromatosis. This type of malignant degeneration of neurofibromatosis occurs in reportedly 5-16% children [1437]. Hence, these patients require long-term follow-up due to risk of recurrence, new tumour formation and malignant transformation.
3.19.3.4. Soft tissue tumours of penis
Mesenchymal tumours are rare in the external genitalia and they require excision in order to differentiate between benign and malignant neoplasms. Histopathological characterisation is essential to ensure malignant tumours receive radical treatment with adjuvant therapy or close follow-up [1427].
Presentation is usually of a painless penile mass, that is non-tender and rubbery on examination. Ultrasound maybe useful in characterising the lesion but is not diagnostic; it can exclude urethral invasion if it is close to urethra [1427]. Once an excision biopsy is performed, if aggressive malignant components are found, a further wider resection may be needed.
Fibrosarcoma is a rare non-rhabdomyosarcoma soft tissue tumour that arises from fibrous tissue. The infantile form of fibrosarcoma is rare and those occurring on the penis are even rarer in the paediatric age-group. Surgical intervention has a favourable prognosis in the paediatric age group with long-term survival of 90% in sporadic cases [1438]. Myofibroma is a benign congenital lesion that occurs either as a solitary lesion or as a part of myofibromatosis with multiple soft tissue tumours. Excision is necessary for histological diagnosis [1427].
Primary penile teratomas are extremely rare subtype of congenital germ cell tumours, and they tend to be asymptomatic and are subdermal on US with no blood flow on Doppler [1439]. They need aggressive treatment with surgical resection due to their unpredictable behavior and unresponsiveness to chemotherapy. Mature teratomas are benign but immature teratoma or even mixed teratomas with immature components can turn malignant and have the potential to metastasise and recur.
3.19.3.5. Penile Lymphedema
Lymphedema in adults is usually secondary to malignancy or infectious disease affecting lymphatic drainage. In the paediatric age group, however, lymphedema is usually primary and generally very rare, affecting 1.2 per 100.000 persons under the age of 20 years [1440]. Of these, only a very small fraction relates to the genital region. Regardless of underlying aetiology, inefficient lymphatic drainage leads to accumulation of subcutaneous lymph which causes tissue swelling and inflammation. This in turn stimulates adipose deposition and fibrosis further exacerbating enlargement. With time the edematous tissue becomes vulnerable to infection, chronic cutaneous changes and disfigurement [1441]. Additionally, when occurring in the genital region urological complications may ensue; such as phimosis, haematuria, bleeding, bladder outlet obstruction, pain, dysuria, lymphorrhea and severe psychological distress due to resultant deformity [1442,1443].
In the largest cohort of male genital oedema in the paediatric age group, 92% of cases were primary; of these only 25% had a discernable familial or syndromic association such as Noonan syndrome, lymphedemadistichiasis or Milroy disease [1442]. Secondary genital lymphedema in children has been reported after inguinal surgery, and non-caseating granulomatous lymphangitis as seen with metastatic Crohn’s disease [1442-1444]. Average age of onset was reported to be 4.5 ± 6.3 years with 61% presenting in infancy, 13% in childhood and the remaining 26% in adolescence. Edema is usually penoscrotal in 72%, isolated scrotal in 24% and very rarely confined exclusively to the penis in 4%. Moreover, concomitant lower limb edema is the rule in two thirds of cases [1442].
There is no general consensus on diagnostic work-up of these patients. History and physical examination (including family history) is usually sufficient. However lymphoscintigraphy can be used as a confirmatory test, more so for limb than genital edema where results can be difficult to interpret [1442]. Ultrasonography is nonspecific, but has been advocated by some to exclude secondary lymphedema by examining the patency of iliac and caval vessels [1445]. Magnetic resonance imaging is useful to exclude other differential diagnoses such as other venous or lymphatic anomalies [1442].
Conservative treatment is the accepted first-line treatment. The mainstay is compression therapy to maintain and prevent further swelling. This can be achieved by compression stockings and undergarments. Additionally, close observation and protection of the skin to prevent excoriations and infection is essential [1442,1445]. Compression therapy is however, less effective on genital oedema than it is on limb edema, especially in growing children. When conservative management fails, and especially in symptomatic cases, or in patients with functional impairment, surgical debulking may be necessary. This can either take the form of circumcision in cases where the foreskin is affected or excision of affected skin and subcutaneous tissues with restructuring and contouring for optimal cosmetic outcome. Complete skin excision and grafting may also be required [1442-1445]. Surgical management can be challenging and needs to be restricted to patients with significant symptoms. Complications include recurrences, continuous lymphatic leakage, haematoma, infection and poor cosmetic outcome [1440,1445,1446].
Summary of evidence | LE |
Cystic penile lesions are the commonest paediatric penile lesions followed by vascular malformations and neurogenic lesions. | 3 |
Neurofibroma patients require long-term followup due to risk of recurrence, new tumour formation and malignant transformation. | 3 |
Mesenchymal tumours are rare and require excision in order to differentiate between benign and malignant neoplasms. | 3 |
Recommendations | LE | Strength rating |
Treatment of penile cystic lesions is by total surgical excision, it is mainly indicated for cosmetic or symptomatic (e.g. infection) reasons. | 4 | Weak |
Propranolol is currently first line treatment for infantile hemangiomas. | 2b | Strong |
Conservative management is the first-line treatment for penile lymphedema. | 4 | Strong |
In symptomatic cases or in patients with functional impairment, surgical intervention may become necessary for penile lymphedema. | 4 | Weak |
3.20. Paediatric urological trauma
Trauma is the leading cause of morbidity and mortality in children and is responsible for more childhood deaths than the total of all other causes [1447]. In about 3% of children seen at paediatric hospital trauma centres, there is significant involvement of the genitourinary tract [1448]. This is caused by either blunt injuries from falls, car accidents, sports injuries, physical assault, sexual abuse, or penetrating injuries, usually due to falls onto sharp objects or from gunshot or knife wounds.
3.20.1. Paediatric renal trauma
3.20.1.1. Epidemiology, aetiology and pathophysiology
In blunt abdominal trauma, the kidney is the most commonly affected organ, accounting for about 10% of all blunt abdominal injuries [1447].
Children are more likely than adults to sustain renal injuries after blunt trauma because of their anatomy. Compared to an adult kidney, a child’s kidney is larger in relation to the rest of the body and often retains foetal lobulations, so that blunt trauma is more likely to lead to a local parenchymal disruption. The paediatric kidney is also less well protected than the adult kidney. Children have less peri-renal fat, much weaker abdominal muscles, and a less ossified and therefore much more elastic and compressible thoracic cage [1449].
Blunt renal trauma is usually a result of sudden deceleration of the child’s body, particularly due to sport accidents, falls, and contact with blunt objects. Deceleration or crush injuries result in contusion, laceration or avulsion of the less well-protected paediatric renal parenchyma.
3.20.1.2. Classification systems
Renal injuries are classified according to the kidney injury scale of the American Association for the Surgery of Trauma (Table 7) [1450].
Table 7: Renal injury classified according to the kidney injury scale of the American Association for the Surgery of Trauma
Grade | Type of injury | Description |
I | Contusion | Non-visible or visible haematuria |
Haematoma | Normal urological studies | |
II | Haematoma | Non-expanding subcapsular haematoma |
Laceration | Laceration of the cortex of < 1.0 cm | |
III | Laceration | Laceration > 1.0 cm without rupture of collecting system |
IV | Laceration | Through the cortex, medulla and collecting system |
Vascular | Vascular injury | |
V | Laceration | Completely shattered kidney |
Vascular | Avulsion of the renal hilum |
3.20.1.3. Diagnostic evaluation
In a child who has sustained blunt abdominal trauma, renal involvement can often be predicted from the history, physical examination and laboratory evaluation. Renal involvement may be associated with abdominal or flank tenderness, lower rib fractures, fractures or vertebral pedicles, trunk contusions and abrasions, and haematuria.
3.20.1.3.1. Haematuria
Haematuria may be a reliable finding. In severe renal injuries, 65% suffer visible haematuria and 33% non-visible, while only 2% have no haematuria at all [1451].
The radiographic evaluation of children with suspected renal trauma remains controversial. Some centres rely on the presence of haematuria to diagnose renal trauma, with a threshold for renal involvement of 50 RBCs/HPF. Although this may be a reliable threshold for significant non-visible haematuria in trauma, there have been many reports of significant renal injuries that manifest with little or even no blood in the urine [1452]. It is therefore compulsory to consider all the clinical aspects involved, including the history, physical examination, consciousness of the child, overall clinical status and laboratory findings to decide on the diagnostic algorithm and whether or not a child needs further imaging studies.
3.20.1.3.2. Blood pressure
It is important to consider that children, unlike adults, are able to maintain their blood pressure, even in the presence of hypovolaemia, due to compliance of the vascular tree and mechanisms for cardiac compensation [1453]. As blood pressure is an unreliable predictor of renal involvement in children, some centres recommend imaging of the urinary tract in children with any degree of haematuria following significant abdominal trauma.
3.20.1.3.3. Choice of imaging method
Nowadays, CT is the best imaging method for renal involvement in children. Computed tomography scanning is the cornerstone of modern staging of blunt renal injuries especially when it comes to grading the severity of renal trauma.
Computed tomography scanning is quite rapid and usually performed with the injection of contrast media. To detect extravasation, a second series of images is necessary since the initial series usually finishes 60 seconds after injection of the contrast material and may therefore fail to detect urinary extravasation. In acute trauma, US may be used as a screening tool and for reliably following the course of renal injury. However, US is of limited value in the initial and acute evaluation of trauma. The standard intravenous pyelogram (IVP) is a good alternative imaging method if a CT scan is not available. It is superior to US but not as good as CT scanning for diagnostic purposes.
3.20.1.4. Disease management
The modern management of trauma is multidisciplinary, requiring paediatricians, emergency physicians, surgeons, urologists, and other specialties as required.
Non-surgical conservative management with bed rest, fluids and monitoring has become the standard approach for treating blunt renal trauma. Even in high-grade renal injuries, a conservative approach is effective and recommended for stable children. However, this approach requires close clinical observation, serial imaging, and frequent re-assessment of the patient’s overall condition. Therefore, a good initial trauma CT with delayed images to check for urinary extravasation is recommended since this may prevent repeat ionising scans. In stable patients with grade 2 or higher lesions a close follow-up with US 48 to 72 hours after the initial scan is sufficient and should be considered before repeating a CT scan [1454]. A systematic review supports application of conservative management protocols also to high-grade blunt paediatric renal trauma. At this time, emergent operative intervention only for haemodynamic instability is recommended. Minimally invasive interventions including angio-embolisation, stenting, and percutaneous drainage should be used when indicated [1455]. Absolute indications for surgery include persistent bleeding into an expanding or unconfined haematoma. Relative indications for surgery are massive urinary extravasation and extensive non-viable renal tissue [1456]. A recently published meta-analysis concluded with the following recommendations: (1) In paediatric patients with blunt renal trauma of all grades, non-operative management vs. operative management in haemodynamically stable patients is strongly recommended. (2) In haemodynamically stable paediatric patients with high-grade (AAST grade III-V) renal injuries, angio-embolisation vs. surgical intervention for ongoing or delayed bleeding is strongly recommended; and, (3) In paediatric patients with renal trauma, routine blood pressure checks to diagnose hypertension is recommended in the long-term follow-up [1457]. However, long-term data on the risk of developing hypertension is lacking.
3.20.1.5. Recommendations for the diagnosis and management of paediatric renal trauma
Recommendations | Strength rating |
Use imaging in all children who have sustained a blunt or penetrating trauma with any level of haematuria, especially when the history reveals a deceleration trauma, direct flank trauma or a fall from a height. | Strong |
Use rapid spiral computed tomography with delayed images scanning for diagnostic and staging purposes. | Strong |
Manage most injured kidneys conservatively. | Strong |
Offer surgical intervention in case of haemodynamic instability and a Grade V renal injury. | Strong |
3.20.2. Paediatric ureteral trauma
Injuries to the ureter are rare. The ureter is well protected; the upper part is protected by its close approximation to the vertebral column and paraspinal muscles and the lower part by its route through the bony pelvis. In addition, the ureter is a small target, and both flexible and mobile. This also means that ureteral injuries are caused more often by penetrating trauma than blunt trauma [1458]. Since the ureter is the sole conduit for urinary transport between the kidney and the bladder, any ureteral injury can threaten the function of the ipsilateral kidney.
3.20.2.1. Diagnostic evaluation
Since there are no classical clinical symptoms suggestive of ureteral trauma, it is important to carry out a careful diagnostic work-up using different imaging modalities. Unfortunately, initial imaging studies, such as IVP and routine CT scans, are unreliable. A study of eleven disruptions of the ureteropelvic junction found that 72% had a normal or non-diagnostic IVP on initial studies [1458]. Diagnostic accuracy of CT scanning can be improved by performing a delayed CT scan up to ten minutes after injection of the contrast material [1459]. The most sensitive diagnostic test is a retrograde pyelogram.
Quite a few patients present several days after the injury, when the urinoma produces flank and abdominal pain, nausea and fever. Due to symptoms being often vague, it is important to remain suspicious of a potential undiagnosed urinary injury following significant blunt abdominal trauma in a child.
3.20.2.2. Management
Immediate repair during abdominal exploration is rare. Minimally invasive procedures are the method of choice, especially since many ureteral injuries are diagnosed late after the traumatic event. Percutaneous or nephrostomy tube drainage of urinomas can be successful, as well as internal stenting of ureteral injuries [1460]. If endoscopic management is not possible, primary repair of partial lacerations should be followed by internal stenting. The management of complete lacerations, avulsions or crush injuries depends on the amount of ureter lost and its location. If there is an adequate healthy length of ureter, a primary ureteroureterostomy can be performed. If primary re-anastomosis is not achievable, distal ureteral injuries can be managed using a psoas bladder hitch, Boari flap or even nephropexy. Proximal injuries can be managed using transureteroureterostomy, auto-transplantation or ureteral replacement with bowel or appendix [1461].
3.20.2.3. Recommendations for the diagnosis and management of paediatric ureteral trauma
Recommendations | Strength rating |
Diagnose suspected ureteral injuries by retrograde pyelogram. | Strong |
Manage ureteral injuries endoscopically, using internal stenting or drainage of an urinoma, either percutaneously or via a nephrostomy tube. | Weak |
3.20.3. Paediatric bladder injuries
The paediatric bladder is less protected than the adult bladder, and is therefore more susceptible to injuries than the adult bladder, especially when it is full, due to:
- Its higher position in the abdomen and its exposure above the bony pelvis.
- The fact that the abdominal wall provides less muscular protection.
- The fact that there is less pelvic and abdominal fat surrounding the bladder to cushion it in trauma.
Blunt trauma is the most common cause of significant bladder injury. In adults, bladder injury is often associated with pelvic fractures. This is less common in children because the paediatric bladder sits above the pelvic ring. In a large prospective study, only 57% of children with pelvic fractures also had a bladder injury compared to 89% of adults [1462].
3.20.3.1. Diagnostic evaluation
The characteristic signs of bladder injury are suprapubic pain and tenderness, an inability to urinate, and visible haematuria (95% of injuries). Patients with a pelvic fracture and visible haematuria present with a bladder rupture in up to 45% of cases [1463].
The diagnosis of bladder rupture can be difficult in some cases. The bladder should be imaged both when fully distended and after drainage using standard radiography or a CT scan. The best results can be achieved by retrograde filling of the bladder using a catheter. Despite advances in CT imaging, the bladder must still be filled to capacity to accurately diagnose a possible bladder injury [1464].
Blunt injuries to the bladder are categorised as:
- contusions with damage to the bladder mucosa or muscle, without loss of bladder wall continuity or extravasation;
- ruptures, which are either intraperitoneal or extraperitoneal.
Intraperitoneal bladder ruptures are more common in children because of the bladder’s exposed position and the acute increase in pressure during trauma. These cause the bladder to burst at its weakest point, i.e. the dome. Extraperitoneal lesions occur in the lower half of the bladder and are almost always associated with pelvic fractures. A cystogram will show extravasation into the perivesical soft tissue in a typical flame pattern and the contrast material is confined to the pelvis.
3.20.3.2. Management
Contusions usually present with varying degrees of haematuria and are treated with catheter drainage alone.
3.20.3.2.1. Intraperitoneal injuries
The accepted management of intraperitoneal bladder ruptures is open surgical exploration and primary repair. Post-operative drainage with a suprapubic tube is mandatory. Recent data suggest that transurethral drainage may be as effective, with fewer complications, resulting in shorter periods of diversion [1465]. Usually, after about seven to ten days, a repeat cystogram is performed to ensure healing is taking place properly.
3.20.3.2.2. Extraperitoneal injuries
Non-operative management with catheter drainage for seven to ten days alone is the method of choice for extraperitoneal bladder rupture. However, if there are bone fragments within the bladder, these must be removed and the bladder must then be repaired and drained, according to the principles for treating intraperitoneal ruptures [1466].
3.20.3.3. Recommendations for the diagnosis and management of paediatric bladder injuries
Recommendations | Strength rating |
Use retrograde cystography to diagnose suspected bladder injuries. | Strong |
Ensure that the bladder has been filled to its full capacity and an additional film is taken after drainage. | Strong |
Manage extra-peritoneal bladder ruptures conservatively with a transurethral catheter left in place for seven to ten days. | Strong |
Do not delay treatment of intra-peritoneal bladder ruptures by surgical exploration and repair as well as post-operative drainage for seven to ten days. | Strong |
3.20.4. Paediatric urethral injuries
Except for the penile part of the urethra, the paediatric urethra is quite well protected. In addition, its shape and elasticity mean the urethra is seldom injured by trauma. However, a urethral injury should be suspected in any patient with a pelvic fracture or significant trauma to the perineum until confirmed otherwise by a diagnostic work-up.
3.20.4.1. Diagnostic evaluation
Patients with suspected urethral trauma and pelvic fractures usually present with a history of severe trauma, often involving other organ systems.
Signs of urethral injury are blood at the meatus, visible haematuria, and pain during voiding or an inability to void. There may also be perineal swelling and haematoma involving the scrotum. A rectal examination to determine the position and fixation of the prostate is important in any male with a suspected urethral injury. The prostate, as well as the bladder, may be displaced up out of the pelvis, especially in membranous urethral trauma.
Radiographic evaluation of the urethra requires a retrograde urethrogram. It is important to expose the entire urethral length, including the bladder neck. If a catheter has already been placed by someone else and there is suspected urethral trauma, the catheter should be left in place and should not be removed. Instead, a small infant feeding tube can be placed into the distal urethra along the catheter to allow the injection of contrast material for a diagnostic scan [1467].
3.20.4.2. Disease management
Since many of these patients are unstable, the urologist’s initial responsibility is to provide a method of draining and monitoring urine output.
A transurethral catheter should only be inserted if there is a history of voiding after the traumatic event, and if a rectal and pelvic examination, as described above, has not suggested a urethral rupture. If the catheter does not pass easily, an immediate retrograde urethrogram should be performed. A suprapubic tube may be placed in the emergency department percutaneously, or even in the operating room, if the patient has to undergo immediate exploration because of other life-threatening injuries.
There are often no associated injuries with a bulbous urethral or straddle injury and management is therefore usually straightforward. In these cases, a transurethral catheter is the best option for preventing urethral bleeding and/or painful voiding [1468].
The initial management of posterior urethral injuries remains controversial, mainly regarding the long-term results with primary realignment compared to simple suprapubic drainage with later reconstruction.
The main goals in the surgical repair of posterior urethral injuries are:
- Providing a stricture-free urethra.
- Avoiding the complications of urinary incontinence and impotence.
Suprapubic drainage and late urethral reconstruction was first attempted because immediate surgical repair had a poor outcome, with significant bleeding and high rates of incontinence (21%) and impotence in up to 56% of cases [1469]. In adults, a study of the success rates of delayed repair reported re-structure rates of 11-30%, continence rates of 90-95% and impotence rates of 62-68% [1470]. However, in children, there is significantly less experience with delayed repair. The largest paediatric series of delayed repair in 68 boys reported a success rate of 90% [1471]. Another study reported strictures and impotence in 67% of boys, although all the boys were continent [1278]. A recently published follow-up study on 15 patients who underwent delayed urethroplasty for blunt urethral trauma during childhood reported high long-term success rates with a low rate of long-term urinary and sexual dysfunction in adulthood [1472].
An alternative to providing initial suprapubic drainage and delayed repair is primary realignment of the urethra via a catheter. The catheter is usually put in place during open cystostomy by passing it from either the bladder neck or meatus and through the injured segment. In a series of fourteen children undergoing this procedure, this resulted in a stricture rate of 29% and incontinence in 7% of patients [1473].
3.20.4.3. Recommendations for the diagnosis and management of paediatric trauma
Recommendations | Strength rating |
Assess the urethra by retrograde urethrogram in case of suspected urethral trauma. | Strong |
Perform a rectal examination to determine the position of the prostate. | Strong |
Manage bulbous urethral injuries conservatively with a transurethral catheter. | Strong |
Manage posterior urethral disruption by either: primary reconstruction; primary drainage with a suprapubic catheter alone and delayed repair; primary re-alignment with a transurethral catheter. | Weak |
3.21. Peri-operative fluid management
3.21.1. Epidemiology, aetiology and pathophysiology
Children have a different total body fluid distribution, renal physiology and electrolyte requirements, as well as weaker cardiovascular compensation mechanisms, compared to adults [1474]. During development, children have a high metabolic rate and lower fat and nutrient stores which means they are more susceptible to metabolic disturbances caused by surgical stress [1475]. The metabolic response to anaesthesia and surgery in infants and children is related to the severity of the operation [1476].
3.21.2. Disease management
3.21.2.1. Pre-operative fasting
Pre-operative fasting has been advocated for elective surgery to avoid the complications associated with pulmonary aspiration during induction of anaesthesia. New regimens include a 30-60 minute limitation for clear liquids [1477,1478] without increased risk of pulmonary aspiration [1479]. Several studies have shown that fasting times in clinical practice often exceed the guidelines with average fasting times of 6-10 hours [1478-1480]. Compared to adults, children have a higher metabolic rate and low glycogen stores and impaired gluconeogenesis, which makes hypoglycaemia an important issue to consider, especially in children < 36 months old [1478]. Therefore, it is important to prevent too long fasting times. Clear-liquid carbohydrate drinks have been proposed to reduce these fasting times [1481].
Table 8 provides the current six, four and one hour guidelines for pre-operative fasting for elective surgery [1478,1480].
Table 8: Pre-operative fasting times for elective surgery
Ingested material | Minimum fasting period (hours) |
Clear liquids | 1 |
Breast milk | 4 |
Light meal | 6 |
3.21.2.2. Maintenance therapy and intra-operative fluid therapy
Generally, the anaesthetist is responsible for intra-operative management and the surgeon is responsible for post-operative instructions. The goal of intra-operative fluid management is to sustain homeostasis by providing the appropriate amount of parenteral fluid; this maintains adequate intravascular volume, cardiac output and oxygen delivery to tissues at a time when normal physiological functions have been altered by surgical stress and anaesthetic agents.
In recent years new strategies for maintenance and replacement fluid management have been developed and this has changed intra-operative fluid management significantly. The main goal of intra-operative fluid management is to maintain a normal extracellular fluid volume (EFV). During the intra-operative period fluid deficits may be induced by blood loss or pre-operative fasting. These fluid deficits can be replaced by balanced isotonic electrolyte solutions to restore a normal EFV. It is recommended that maintenance intravenuous (IV) fluids should consist of balanced isotonic solutions with appropriate potassium chloride and dextrose in order to decrease the risk of hyponatraemia development [1482]. No increased risk for hypernatraemia, fluid overload with aedema and hypertension, and hyperchloremic acidosis was found, which was always feared for isotonic solutions [1482].
When children are clinically unstable due to third-space losses, these losses should be replaced with crystalloids (normal saline or Ringer’s lactate). Third-space losses may vary from 1 mL/kg/h for a minor surgical procedure to 15-20 mL/kg/h for major abdominal procedures, or even up to 50 mL/kg/h for surgery of necrotising enterocolitis in premature infants. When this fluid management is insufficient replacement management with colloids (albumin, gelatine and hydroxyethyl starch [HES]) should be adopted, using a restrictive approach [1483].
Clinical guidelines have been proposed by Sümpelmann et al., [1483] regarding intra-operative fluid management (Table 9).
Table 9: Intra-operative fluid management
Solution for infusion | Initial/repeated dose | |
Background infusion | Balanced isotonic solution + 1-2% glucose | 10 mL/kg/h |
Fluid therapy | Balanced isotonic solution | X 10-20 mL/kg |
Volume therapy | Albumin, Gelatine, hydroxyethyl starch | X 5-10 mL/kg |
Transfusion | Red blood cells, fresh frozen plasma, platelets | X 10 mL/kg |
3.21.2.3. Post-operative feeding and fluid management
It is not obligatory to check serum chemistry after uncomplicated surgery in children with normal pre-operative renal and hepatic function. However, if oral intake has been postponed for > 24 hours (e.g. as in intestinal surgery), there is an increased risk of electrolyte abnormalities, requiring further assessment and subsequent management, particularly with potassium. Post-operative findings, such as decreased bowel movements and ileus, may be signs of hypokalaemia.
Children who undergo interventions to relieve any kind of obstructive diseases deserve particular attention, especially due to the risk of polyuria as a result of post-obstructive diuresis [1484]. In children who develop polyuria, it is important to monitor fluid intake and urine output, as well as renal function and serum electrolytes. If necessary, clinicians should not hesitate in consulting with a paediatric nephrologist.
In children who have undergone non-abdominal surgery, studies have suggested that gastric motility returns to normal one hour after emergence from anaesthesia [1485]. Early post-operative intake of fluid in children who have undergone minor or non-abdominal urological surgery is associated with reduced post-operative vomiting and lower opioid use [1486] and is therefore encouraged.
In abdominal surgery the enhanced recovery after surgery (ERAS) protocol has been implemented in the paediatric population following its success in adults [1481,1487]. The ERAS protocol is a multimodal approach to prevent the post-operative effects of the surgical stress response. This protocol includes pre- and intraoperative element such as minimal pre-operative fasting and careful intra-operative fluid management, and also focuses on post-operative care. The post-operative ERAS protocol suggests starting clear fluid intake on the evening of surgery and a normal diet the day after surgery and thereby early discontinuation of IV fluids. Further focus is on early mobilisation, preventing epidurals and omitting or early removal of external tubes [1481,1487].
The implementation of an ERAS protocol has resulted in shorter length of hospital stays, faster bowel recovery and opioid-free post-operative need [1481,1487,1488]. When implementing ERAS in children with neurological abnormalities special attention should be given to bowel management with pre-operative treatment of constipation and early post-operative continuation of routine bowel management.
3.21.3. Summary of evidence and recommendations for the management of peri-operative fluid management
Summary of evidence | LE |
Children are not simply smaller physiological versions of adults. They have their own unique metabolic features, which must be considered during surgery. | 2 |
During the intra-operative period balanced isotonic electrolyte solutions can be used to maintain a normal extracellular fluid volume. | 1 |
Following abdominal surgery ERAS protocols can be used to reduce recovery times and complications. | 1 |
Recommendations | Strength rating |
Ensure shorter pre-operative fasting periods for elective surgeries (up to one hour for clear liquids). | Strong |
Use enhanced recovery after surgery protocols for abdominal surgery in children with normal bowel movement. | Strong |
Use isotonic solutions in hospitalised children because they are at high risk of developing hyponatraemia. | Strong |
Assess the baseline and daily levels of serum electrolytes, glucose, urea and/or creatinine in every child who receives intravenous fluids, especially in intestinal surgery (e.g. ileal augmentation), regardless of the type of solution chosen since there is an increased risk of electrolyte abnormalities in children undergoing such surgery. | Strong |
Start early oral fluid intake in all patients scheduled for minor surgical procedures. | Strong |
3.22. Post-operative pain management: general information
3.22.1. Epidemiology, aetiology and pathophysiology
The provision of adequate pain control requires proper pain evaluation, accurate choice of drug and route of administration, and consideration of age, physical condition and type of surgery and anaesthesia [1489].
Traditional medical beliefs that neonates are incapable of experiencing pain have now been abandoned following recent and better understanding of how the pain system matures in humans, better pain assessment methods and a knowledge of the clinical consequences of pain in neonates [1490,1491]. Many studies have indicated that deficient or insufficient analgesia may be the cause of future behavioural and somatic sequelae [1492,1493]. Our current understanding of pain management in children depends fully on the belief that all children, irrespective of age, require adequate pain treatment.
3.22.2. Diagnostic evaluation
Assessment of pain is the first step in pain management. Several pain assessment tools have been validated according to the child’s age, cultural background, mental status, communication skills and physiological reactions [1494]. Depending on the child’s age, the 0-10 Numeric Rating Scale, Faces Revised Pain Scale or Colour Analog Scale, for example, can be used [1495]. One of the most important topics in paediatric pain management is informing and involving the child and caregivers during this process. Patient-family-controlled-analgesia is the preferred pain management in the hospital and at home if provided with the correct information [1495,1496].
3.22.3. Disease management
3.22.3.1. Drugs and route of administration
Pre-emptive analgesia is an important concept that aims to induce the suppression of pain before neural hypersensitisation occurs [1497]. Regional anaesthesia are given intra-operatively which can include a regional nerve block, caudal blocks or local wound infiltration and has proven to reduce the need for post-operative analgesia [1498]. The WHO’s ‘pain ladder’ is a useful tool for the pain management strategy [1499]. A three level strategy seems practical for clinical use. Post-operative management should be based on sufficient intraoperative pre-emptive analgesia with regional or caudal blockade followed by balanced analgesia. Paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs) are the drugs of choice at the first level. As they become insufficient to prevent pain, weak and strong opioids are added to oral drugs to achieve balanced analgesia. Every institute must build their own strategy for post-operative analgesia. A proposed strategy for postoperative analgesia may be as follows:
1.Intra-operative regional or caudal block.
3.Paracetamol + NSAID + weak opioid (e.g. tramadol or codeine).
4.Paracetamol + NSAID + strong opioid (e.g. morphine, fentanyl, oxycodone or pethidine).
The use of opioids in children has long held a standard role in the post-operative management of pain. Increased recognition of the adverse effects of opioids and prolonged opioid dependency demand a balanced intra-operative administration of opioids [1495,1500]. Intra-operative adequate dosage of paracetamol and NSAIDs results in a decrease in opioid requirement in children [1501,1502]. Furthermore, opioid awareness among physicians could reduce opioid use. When prescribing lower opioid dosage, this did not increase pain scores in urological outpatient surgeries [1503]. Caution is necessary to take account of renal function when using NSAIDs. Paediatric dependent dosages for most common used pain medication can be found in this publication [1504].
3.22.3.2. Circumcision
Circumcision requires anaesthesia and proper pain management [1505]. Potential analgesic interventions during circumcision include the use of a dorsal penile nerve block (DPNB) or ring block, topical anaesthetics (e.g. lidocaine-prilocaine cream, or 4% liposomal lidocaine cream), and sucrose preferably in combination [1498,1504]. Caudal blockade methods have similar efficacy compared to DPNB. However, caregivers should be informed about the more frequent incidence of post-operative motor weakness and micturition problems [1506]. Ultrasound guidance can be used [1504].
3.22.3.2.1. Penile, inguinal and scrotal surgery
Caudal blocks and peripheral nerve blocks (DPNB and pudendal) are commonly used methods for analgesia following surgery for hypospadias. Several agents with different doses, concentrations and administration techniques have been used and shown to be adequate. Overall post-operative pain scores were lower with pudendal nerve blocks. No increase in post-operative complications was seen with these types of blocks [1498,1507,1508]. Severe bladder spasms caused by the presence of the bladder catheter may sometimes cause more problems than pain and is managed with antimuscarinic medications. For inguinoscrotal surgery, various regional anaesthesia methods have been investigated, such as transversus abdominis plane block, ilioinguinal/iliohypogastric nerve blocks and caudal blocks. All have been shown to have adequate postoperative analgesic properties. Additional local anaesthetics such as clonidine or dexmedetomidine may improve results [1498].
3.22.3.3. Bladder and kidney surgery
Continuous local infusion reduces the need for post-operative opioids [1509-1511], as well as systemic (intravenous) application of analgesics [1512], has been shown to be effective. Ketorolac is an effective agent that is underused. It decreases the frequency and severity of bladder spasms and the length of post-operative hospital stay and costs [1513,1514]. Open kidney surgery is particularly painful because all three muscle layers are cut during conventional loin incision. A dorsal lumbotomy incision may be a good alternative because of the shorter post-operative hospital stay and earlier return to oral intake and unrestricted daily activity [1515]. Caudal and paravertebral blocks continuous epidural analgesia, as well as rectus sheath and transversus abdominis plane blocks have decreased post-operative morphine requirement after abdominal and renal surgery [1516-1518]. For laparoscopic approaches, intra-peritoneal spraying of local anaesthetic before incision of the perirenal fascia may be beneficial [1519].
3.22.4. Summary of evidence and recommendations for the management of post-operative pain
Summary of evidence | LE |
Adequate paracetamol and NSAIDs use reduces opioid need post-operatively. | 1 |
Pain may cause behavioural and somatic sequelae. | 3 |
Every institute must develop their own well-structured strategy for post-operative analgesia. | 4 |
Recommendations | Strength rating |
Prevent/treat pain in children of all ages. | Strong |
Evaluate pain using age-compatible assessment tools. | Strong |
Inform patients and caregivers accurately. | Strong |
Use pre-emptive and balanced analgesia in order to decrease the side effects of opioids. | Strong |
3.23. Basic principles of laparoscopic surgery in children
3.23.1. Epidemiology, aetiology and pathophysiology
The use of laparoscopy and robot-assisted laparoscopic surgery is rapidly increasing and has gained widespread acceptance for many urological surgeries in children. Diagnostic laparoscopy for undescended testis, nephrectomy, heminephrectomy, varicocelectomy, pyeloplasty and ureteral reimplantation are some of the indications which are commonly being performed. This expanding scope related to technological advancements allows surgeons to perform more complex procedures in a minimally invasive fashion even in infants and younger children. Generally, well established benefits of minimally invasive surgery are decreased pain, shorter convalescence and better cosmetics compared to traditional open surgery [876]. Additional advantages of robotic surgery over conventional laparoscopy include ergonomics, 3D vision, better manoeuvrability, decreased tremor and easy learning curve. Limitations to be considered are increased operative time, smaller working space at young age, cost and experience of the surgeon and anaesthesiologist. While the success and complication rates are comparable for nephrectomy and pyeloplasty (see chapter 3.13.3.2) advantages of laparoscopy and robotic surgery for ureteral reimplantation have not been proven and this can only be recommended for experienced centres (see chapter 3.14.3.2.3).
As worldwide experience increases, there is an accumulating awareness about the physiological consequences related to intra- and retroperitoneal carbon dioxide (CO2) insufflation in children. In contrast to traditional open surgery pneumoperitoneum may have physiological responses which require close monitoring during surgery and should be taken seriously.
3.23.2. Technical considerations and physiological consequences
3.23.2.1. Pre-operative evaluation
Laparoscopy in children requires specific anaesthetic precautions. Physiological effects of CO2 pneumoperitoneum, positioning of the patient and in potentially increased operative time need to be considered by the anaesthesiology team. Therefore, a detailed medical examination and risk assessment is mandatory pre-operatively. Especially cardiac and pulmonary system should be assessed since increased intra-abdominal pressure may lead to decreased ventricular preload [1520].
3.23.2.2. Abdominal insufflation
Abdominal insufflation is the main principle of laparoscopic surgery to create working space for the surgeon. Carbon dioxide is the most commonly used insufflant in laparoscopic centres throughout the world. Other alternatives reported are nitrous oxide, helium, argon and air. However, CO2 is considered to be the best available gas as it is colourless, cheap, has high solubility in the vascular system [1521] and is excreted by the pulmonary system making it the safest option. Smaller children and infants absorb more CO2 than older children [1522], suggesting the need for more attention both during and early after laparoscopic surgery for these children.
Most complications of laparoscopy are attributable to gaining access to the abdominal cavity. One study reporting complications of > 5,400 paediatric laparoscopic surgeries showed that there was an overall complication rate of 5.3% of which 4.2% were related to problematic insufflation (subcutaneous emphysema, gas embolism, injury to the organs and vascular structures, mis-insufflation etc.) [1523]. There are two main and well-established techniques for initial access to the abdomen or retroperitoneum: open technique (Hasson) and Veress needle. Studies comparing these two different access techniques in paediatric laparoscopic urological procedures showed similar complication rates [1524]. The vast majority of the complications were minor and related to lack of surgical experience. Particularly in infants and smaller children, the open access technique is recommended by the Panel to reduce the chance of complications.
Elasticity of the abdominal wall is age-related and is higher in infants and small children compared to older children [1525].
Pneumoperitoneal pressure (PnP in mmHg) is one of the critical points that needs to be carefully considered by laparoscopic surgeons. A recent RCT compared two different pneumoperitoneal pressure groups (6-8 mmHg vs. 9-10 mmHg) in infants less than 10 kg [1526]. It demonstrated that higher pressures were associated with more pronounced respiratory and haemodynamic changes as well as increased post-operative pain scores and prolonged time to resume feeding.
3.23.2.3. Pulmonary effects
After intra-abdominal insufflation the diaphragm is pushed upwards due to increased abdominal pressure. This leads to decreased total pulmonary compliance. Combined with CO2 absorption this may lead to hypercarbia and acidosis, particularly in case of prolonged operative time or low pulmonary reserve such as in infants. Trendelenburg position may also aggravate the situation in operations in the pelvic region, such as anti-reflux or bladder neck surgeries. Several studies revealed increased end tidal CO2 (ET CO2) related to CO2 absorption [1522,1527,1528]. One study showed a 33% increase in ET CO2 in the majority of neonatal laparoscopic and thoracoscopic procedures [1336]. Shorter operative time and lower intra-abdominal pressures decrease the risk of increased ET CO2. Hypoxemia is rarely seen, even in neonates and can easily be adjusted by increasing minute ventilation. These findings highlight the importance of close monitoring of the children.
3.23.2.4. Cardiovascular effects
Intra-abdominal pressure, CO2 absorption and positioning may also affect the cardiovascular system. It has been shown in adults that after initiation of pneumoperitoneum, cardiac output and stroke volume decrease while mean arterial pressure, central venous pressure and systemic vascular resistance increase [1529]. Similar outcomes have been reported during paediatric laparoscopy with some nuances. Cardiac output was 30% decreased while blood pressure remained stable during laparoscopic orchidopexy with PnP of 10 mmHg in children between aged 6-30 months [1530]. When PnP was lowered from 12 mmHg to 6 mmHg, cardiac index and other vascular parameters normalised [1531]. Using high intra-abdominal pressures in infants with congenital cardiac abnormalities may result in re-opening of cardiac shunts such as the foramen ovale and ductus arteriosus [1532]. Although cardiovascular effects of using high PnP are clinically measurable, they may not have a significant clinical impact on healthy children. However, it is clear that using lower pressures is safer especially in smaller children.
3.23.2.5. Effects on renal function
Although clinical studies in children are lacking, pneumoperitoneum may also have adverse effects on renal blood flow [1533]. High intra-abdominal pressures and reverse Trendelenburg position may cause decreased glomerular filtration rate and decreased urine output. One study has shown that 88% of infants and 14% of children more than one year old develop anuria within 45 minutes after initiation of PnP with 8 mmHg [1534]. However, urine output recovers with temporary polyuria after the operation. Although the clinical relevance of decreased urine output seems insignificant, it is important to monitor the fluid and electrolyte balance of the children during and after laparoscopic surgery.
3.23.2.6. Effects on neurological system
Another effect of pneumoperitoneum is increased intracranial pressure (ICP) which normalises after desufflation of the abdomen [1535]. Trendelenburg position, high PnP and hypoventilation are additional risk factors for increased ICP. Laparoscopy is therefore contraindicated in patients with intracranial space occupying lesions [1536]. Children with ventriculo-peritoneal shunts require precautions with regards to shunt drainage, however laparoscopy is not contraindicated [1537].
3.23.3. Summary of evidence and recommendations for laparoscopy in children
Summary of evidence | LE |
Laparoscopy and robotic-assisted laparoscopic surgery can safely be performed in children | 1 |
The general benefits of laparoscopy are decreased pain, shorter convalescence and better cosmetics compared to traditional open surgery. | 1 |
Limitations to be considered are increased operative time, smaller working space with young age, cost, surgeon and anaesthesiologist experience. | 1 |
Pneumoperitoneum may have physiological effects which require close monitoring during surgery and should be taken seriously. | 2 |
Recommendations | Strength rating |
Use lower intra-abdominal pressure (6-8 mmHg) during laparoscopic surgery in infants and smaller children. | Strong |
Use open access for laparoscopy in infants and smaller children. | Strong |
Monitor for laparoscopy-related cardiac, pulmonary and diuretic responses. | Strong |