5. DIAGNOSTIC EVALUATION
5.1. Screening and individual early detection
5.1.1. Screening
Population or mass screening is defined as the ‘systematic examination of asymptomatic men to identify individuals at risk for a specific disease’ and is usually initiated by health authorities. The co-primary objectives are:
- reduction in mortality due to PCa;
- a maintained QoL as expressed by QoL-adjusted gain in life years (QALYs).
Screening for PCa still is one of the most controversial topics in the urological literature [131]. A Cochrane review of randomised PCa screening trials with PCa mortality as endpoint was published in 2013 [132] and updated in 2018 [133,134]. The main findings of the updated publication from the results of 5 RCTs, randomising more than 721,718 men, are:
- Screening is associated with an increased diagnosis of PCa (Incidence ratio [IR]: 1.23 95% CI: 1.03–1.48).
- Screening is associated with detection of more localised disease (RR: 1.39, [1.09–1.79]) and less advanced PCa (T3–4, N1, M1; RR: 0.85 [0.72–0.99]).
- No PCa-specific survival benefit was observed (IR: 0.96 [0.85–1.08]). This was the main endpoint in all trials.
- No overall survival (OS) benefit was observed (IR: 0.99, 95% CI: 0.98–1.01). None of the trials were designed/powered for this endpoint.
The included studies are different regarding multiple aspects, including: trial size, time periods, age groups, participation/compliance rates, previous screening rates (opportunistic testing in control arm, ‘contamination’), one-time vs. repeat screening, and the applied diagnostic pathway. These differences account for discrepancies in results between single studies and the Cochrane review aggregated findings.
The ERSPC (European Randomized Study of Screening for Prostate Cancer) started in the early 90’s, included >182K European men, found a significant reduction in PCa mortality due to screening. ERSPC applied a mainly PSA-based screening protocol (cut-off 3.0–4.0 ng/mL followed by systematic sextant prostate biopsy, every 2–4 years in men aged 50–74). The contamination rate was relatively low when compared to other large studies such as the PLCO (Prostate Lung Colorectal & Ovarian) screening trial [135]. A limitation is the heterogenity in patient groups and the applied screening protocols. Since 2013, data have been updated with 16 years of follow-up [135]. With extended follow-up, the mortality reduction (21% and 29% after non-compliance adjustment) remains unchanged. However, the number needed to screen (NNS) and to treat is decreasing and is now below the NNS observed in breast cancer trials [135,136] (Table 5.1).
Table 5.1: Follow-up data from the ERSPC study [135]
Years of follow-up | Number needed to screen | Number needed to treat |
9 | 1,410 | 48 |
11 | 979 | 35 |
13 | 781 | 27 |
16 | 570 | 18 |
In the Göteborg screening trial, with 18 years of follow-up, the ratio of death from PCa for the screening group compared with the control group was 0.65 (95% CI: 0.49–0.87) and for men starting screening at age 55–59 it was 0.47 (95% CI: 0.29–0.78) [137]. The number needed to invite was 231; the number needed to diagnose 10.
A comparison of systematic and opportunistic screening suggested over-diagnosis and mortality reduction in the systematic screening group compared to a higher over-diagnosis with only a marginal survival benefit, at best, in the opportunistic screening regimen [138].
The benefit of screening in reducing PCa-specific mortality (PCSM) and the even more favourable impact on metastases rates, is counter-balanced by the side effects of screening such as increased diagnosis rates, which has led to over-treatment of mainly low-risk PCa, and subsequent treatment-related effects [139]. Regarding QoL, the beneficial effects of screening and the side effects seem to balance out, resulting in limited overall impact on the invited population [139-141].
National USA recommendations against PSA-based screening resulted in a reduction in the use of PSA for early detection and was associated with higher rates of advanced disease [11,112,142-147]. Initial widespread aggressive screening in USA was associated with a decrease in PCa mortality, which has decreased for two decades since the introduction of PSA testing [148-150]. The current USA national recommendation for men
< 70 years of age is that the decision to be screened should be an individual one [151-153].
Recognition of the harms of over-diagnosis and over-treatment had led to a redesign in the pathway for early detection of PCa including identification of specific risk groups, individualised re-testing interval, improved indication for biopsy using risk calculators and/or MRI, targeted biopsies, and the application of AS for low-risk disease.
The inclusion of MRI may improve a screening protocol, as it reduces the number of men that undergo biopsies while detecting more high-grade and less low-grade PCa [154-156]. The Stockholm-3 (STHLM3) screening trial randomised men with a PSA > 3 ng/mL between standard biopsies (10–12 cores) or MRI and standard plus targeted biopsies in the presence of a suspicious MRI. The percentage of men that underwent prostate biopsies in the standard group was double that of the MRI group. In this non-inferiority trial, the intention-to-treat (ITT) analysis found 18% and 21% clinically significant disease (ISUP Grade group > 1) and 12% and 4% insignificant disease in the standard and the MRI group, respectively [154]. The IP1-PROSTAGRAM study (PSA > 3 ng/mL; MRI Prostate Imaging – Reporting and Data System [PI-RADS] > 2), showed highest detection of csPCa for MRI compared to transrectal ultrasound-guided prostate (TRUS) biopsy in a population screening setting [155].
The Identification of Men With a Genetic Predisposition to ProstAte Cancer (IMPACT) study, evaluates targeted PCa screening using PSA in men aged 40–69 years with germline BRCA1/2 mutations (annually, biopsy recommended if PSA > 3.0 ng/mL). After 3 years of screening, BRCA2 mutation carriers were associated with a higher incidence of PCa, a younger age of diagnosis, and more clinically significant tumours compared with non-carriers [26,157]. The influence of BRCA1 mutations on PCa remained unclear. No differences in age or tumour characteristics were detected between BRCA1 carriers and BRCA1 non-carriers. The mismatch repair cohort of IMPACT in men with MSH2 and MSH6 pathogenic variants found a higher incidence of significant PCa vs. non-carriers [158].
5.1.2. Individual early detection
Early detection may be initiated on an individual level in men with risk factors (age > 50; men from 45 years of age and a family history of PCa; men of African descent from 45 years of age; men carrying BRCA2 mutations from 40 years of age). Decreased disease-specific mortality on one side, but increased incidence with the risk of over-treatment on the other side, should be discussed with men.
5.1.2.1. Risk assessment, co-morbidity and life-expectancy
Data from the Goteborg arm of the ERSPC trial suggest that the age at which early diagnosis should be stopped remains controversial, but an individual’s life expectancy must definitely be taken into account. Men who have less than a 15-year life expectancy are unlikely to benefit, based on data from the Prostate Cancer Intervention Versus Observation Trial (PIVOT) and the ERSPC trials. Furthermore, although there is no simple tool to evaluate individual life expectancy, co-morbidity is at least as important as age. A detailed review can be found in Section 5.4 ‘Estimating life expectancy and health status’ and in the SIOG Guidelines [159].
5.1.2.2. Initial risk assessment by PSA and DRE
Informed men requesting an early diagnosis should be given a PSA test and undergo a DRE [160]. The use of DRE alone in the primary care setting had a sensitivity and specificity below 60%, possibly due to inexperience, and can therefore not be recommended to exclude PCa [161]. PSA measurement and DRE need to be repeated [162], but the optimal intervals for PSA testing and DRE follow-up are unknown as they varied between several prospective screening trials. A risk-adapted strategy might be a consideration, based on the initial PSA level. Men with a baseline PSA < 1 ng/mL at 40 years and < 2 ng/mL at 60 years are at decreased risk of PCa metastasis or death from PCa several decades later [50,163]. The retesting interval can therefore be every 2 years for those initially at risk, or postponed up to 8 years in those not at risk with an initial PSA < 1 ng/mL at 40 years and a PSA < 2 ng/mL at 60 years of age and a negative family history [164]. An analysis of ERSPC data supports a recommendation for an 8-year screening interval in men with an initial PSA concentration < 1 ng/mL; fewer than 1% of men with an initial PSA concentration < 1 ng/mL were found to have a concentration above the biopsy threshold of 3 ng/mL at 4-year follow-up; the cancer detection rate by 8 years was close to 1% [165]. The long-term survival and QoL benefits of extended PSA re-testing (every
8 years) remain to be proven at a population level.
5.1.2.3. Risk assessment to determine the need for biopsy
Multiple diagnostic tools are now available to determine the need for a biopsy to establish the diagnosis of a PCa.
Risk calculators, combining clinical data (age, DRE findings, PSA level, prostate volume, etc.) may be useful in helping to determine (on an individual basis) what the potential risk of cancer may be, thereby reducing the number of unnecessary biopsies. The risk calculator selected should have been calibrated to the target population; lack of calibration might represent a real limiting factor for its use [166]. Several tools developed from cohort studies are available including (among others):
- the ERSPC cohort: http://www.prostatecancer-riskcalculator.com/seven-prostate-cancer-risk-calculators; This calculator has been updated by incorporating the 2014 ISUP Pathology Gleason Grading and Cribriform growth [167];
- the PCPT cohort: PCPTRC 2.0 http://myprostatecancerrisk.com/.
Prostate MRI stratifies patients with an indication for biopsy on a 1- to 5- risk scale of having csPCa. Prostate MRI and related MRI-directed biopsies have shown to be at least as diagnostically effective as systematic biopsies alone in diagnosing significant cancers [168] (see Section 5.2.4.2.4).
Moreover, in a prospective, multi-centre, non-randomised opportunistic early detection setting (PSA > 3 ng/mL), the MRI-directed biopsy decision strategy avoided more men biopsied in comparison to a diagnostic pathway using a risk calculator and then systematic biopsy (559/1015, 55% vs 403/950, 42%; difference -13%, 95% CI: -17% to -8.3%; p < 0.01); it also detected less ISUP grade 1 cancers (84/1015, 8.3% vs. 121/950, 13%; difference 4.5%, 95% CI: 1.8–7.2%; p < 0.01) [169].
PSA-density (PSA-D) is the strongest predictor in risk calculators. Combinations of PSA-D and MRI have been explored [170-175], showing guidance in biopsy-decisions whilst safely avoiding redundant biopsy testing (see Section 5.2.4.2.6.3).
Urine and serum biomarkers as well as tissue-based biomarkers have been proposed for improving detection and risk stratification of PCa patients, potentially avoiding unnecessary biopsies. However, further studies are necessary to validate their efficacy [176]. At present there is too limited data to implement these markers into routine screening protocols (see Section 5.2.3).
5.1.3. Genetic testing for inherited prostate cancer
Increasing evidence supports the implementation of genetic counselling and germline testing in early detection and PCa management [177]. Several commercial screening panels are now available to assess main PCa risk genes [178]. However, it remains unclear when germline testing should be considered and how this may impact localised and metastatic disease management. Germline BRCA1 and BRCA2 mutations occur in approximately 0.2% to 0.3% of the general population [179]. It is important to understand the difference between somatic testing, which is performed on the tumour, and germline testing, which is performed on blood or saliva and identifies inherited mutations. Genetic counselling is required prior to and after undergoing germline testing.
Germline mutations can drive the development of aggressive PCa. Therefore, the consensus is the following men, with a personal or family history of PCa or other cancer types arising from DNA repair gene mutations should be considered for germline testing:
- Men with metastatic PCa;
- Men with high-risk PCa and a family member diagnosed with PCa at age < 60 years;
- Men with multiple family members diagnosed with csPCa at age < 60 years or a family member who died from PCa cancer;
- Men with a family history of high-risk germline mutations or a family history of multiple cancers on the same side of the family.
Further research in this field (including not so well-known germline mutations) is needed to develop screening, early detection and treatment paradigms for mutation carriers and family members.
5.1.4. Guidelines for germline testing*
Recommendations | Strength rating |
Consider germline testing in men with metastatic PCa. | Weak |
Consider germline testing in men with high-risk PCa who have a family member diagnosed with PCa at age < 60 years. | Weak |
Consider germline testing in men with multiple family members diagnosed with PCa at age | Weak |
Consider germline testing in men with a family history of high-risk germline mutations or a family history of multiple cancers on the same side of the family. | Weak |
*Genetic counselling is required prior to germline testing.
5.1.5. Guidelines for screening and individual early detection
Recommendations | Strength rating |
Do not subject men to prostate-specific antigen (PSA) testing without counselling them on the potential risks and benefits. | Strong |
Offer an individualised risk-adapted strategy for early detection to a well-informed man with a life-expectancy of at least 10 to 15 years. | Weak |
Offer early PSA testing to well-informed men at elevated risk of having PCa:
| Strong |
Offer a risk-adapted strategy (based on initial PSA level), with follow-up intervals of 2 years for those initially at risk:
Postpone follow-up up to 8 years in those not at risk. | Weak |
In asymptomatic men with a prostate-specific antigen (PSA) level between 3–10 ng/mL and a normal digital rectal examination (DRE), repeat the PSA test prior to further investigations. | Weak |
In asymptomatic men with a PSA level between 3–10 ng/mL and a normal DRE, use one of the following tools for biopsy indication:
| Strong |
| Weak |
Stop early diagnosis of PCa based on life expectancy and performance status; men who have a life-expectancy of < 15 years are unlikely to benefit. | Strong |
5.2. Clinical diagnosis
Prostate cancer is usually suspected on the basis of DRE and/or PSA levels. Definitive diagnosis depends on histopathological verification of adenocarcinoma in prostate biopsy cores.
5.2.1. Digital rectal examination
In ~18% of cases, PCa is detected by suspect DRE alone, irrespective of PSA level [180]. A suspect DRE in patients with a PSA level < 2 ng/mL has a positive predictive value (PPV) of 5–30% [180]. In the ERSPC trial, an abnormal DRE in conjunction with an elevated PSA more than doubled the risk of a positive biopsy (48.6% vs. 22.4%) [181]]. An abnormal DRE is associated with an increased risk of a higher ISUP grade, predicts csPCa in men under AS [182] and is an indication for MRI and biopsy [181,183]. cT staging is dependent on DRE and a strong predictor of advanced PCa (OR: 11.12 for cT3 and OR: 5.28 for cT4) [184].
5.2.2. Prostate-specific antigen
The use of PSA as a serum marker has revolutionised PCa diagnosis [185]. Prostate-specific antigen is organ- but not cancer specific; therefore, it may be elevated in benign prostatic hypertrophy (BPH), prostatitis and other non-malignant conditions. However, PSA keeps its diagnostic value for cancer detection even in symptomatic patients [186].
PSA value is also negatively impacted by hormonal treatments, even those given for benign conditions such as finasteride or dutasteride [187]. In such cases, interpretation of PSA level should be adapted as these treatments can frequently lower the PSA level by half.
There are no agreed standards for defining PSA thresholds [188]. It is a continuous parameter, with higher levels indicating greater likelihood of PCa. Many men may harbour PCa despite having low serum PSA [189]. Table 5.2 demonstrates the occurrence of ISUP > grade 2 PCa in systematic biopsies at low PSA levels, precluding an optimal PSA threshold for detecting non-palpable but csPCa. The use of nomograms and biomarkers may help in predicting indolent PCa [154,190,191]. In case of an elevated PSA (up to 10 ng/mL), a repeated test should be considered to confirm the increase before going to the next step.
5.2.2.1. Repeat PSA testing
A repeat PSA test before prostate biopsies in men with an initial PSA 3–10 ng/mL reduced the indication for biopsies in 16.8% of men while missing 5.4% ISUP grade > 1 in the STHLM3 trial [192]. Similarly, in the Prostate Testing for Cancer and Treatment (ProtecT) trial men with a more than 20% lower repeat-PSA analysis within 7 weeks had a lower risk of PCa (OR: 0.43, 95% CI: 0.35–0.52) as well as a lower risk of ISUP grade > 2 (OR: 0.29, 95% CI: 0.19–0.44) [193]. A study with a PSA interval of 4 weeks showed similar findings of a reduced risk of PCa and ISUP grade > 1 [194]. These observations indicate that an early repeat-PSA prior to the decision of prostate biopsies has prognostic information.
Table 5.2: Risk of PCa identified by systemic PCa biopsy in relation to low PSA values
PSA level (ng/mL) | Risk of PCa (%) | Risk of ISUP grade > 2 PCa (%) |
0.0–0.5 | 6.6 | 0.8 |
0.6–1.0 | 10.1 | 1.0 |
1.1–2.0 | 17.0 | 2.0 |
2.1–3.0 | 23.9 | 4.6 |
3.1–4.0 | 26.9 | 6.7 |
5.2.2.2. PSA density
Prostate-specific antigen density is the level of serum PSA divided by the prostate volume. The higher the PSA-D, the more likely it is that the PCa is clinically significant; in particular in smaller prostates when a PSA-D cut-off of 0.15 ng/mL/cc was applied [195] (see Section 5.2.4.2.6.3). Several studies found a PSA-D over 0.1–0.15 ng/mL/cc predictive of cancer [196,197]. Patients with a PSA-D below 0.09 ng/mL/cc were found unlikely (4%) to be diagnosed with csPCa [198]. A systematic review showed heterogeneity among studies using PSA-D to select men with PI-RADS 3 category on MRI reading for biopsies but suggest a cut-off of 0.15 ng/mL/cc [196]. Others found its added value to biparametric (bp) MRI-guided biopsies unclear with an area under the curve (AUC) of 0.87–0.95 for the direction of csPCa based on bpMRI and 0.91–0.95 for the combined test of bpMRI and PSA-D [199].
5.2.2.3. Free/total PSA ratio
Free/total (f/t) PSA must be used cautiously because it may be adversely affected by several pre-analytical and clinical factors (e.g., instability of free PSA at 4°C and room temperature, variable assay characteristics, and concomitant BPH in large prostates) [200]. A systematic review including 14 studies found a pooled sensitivity of 70% in men with a PSA of 4–10 ng/mL [201]. Free/total PSA is of no clinical use if the total serum PSA is > 10 ng/mL or during follow-up of known PCa. The clinical value of f/t PSA is limited in light of the new diagnostic pathways incorporating MRI.
5.2.3. Other blood and urine biomarkers
5.2.3.1. Blood based biomarkers: PHI/4K score/IsoPSA
Several assays measuring a panel of kallikreins in serum or plasma are now commercially available, including the U.S. Food and Drug Administration (FDA) approved Prostate Health Index (PHI) test (combining free and total PSA and the [-2]pro-PSA isoform [p2PSA]), and the four kallikrein (4K) score test (measuring free, intact and total PSA and kallikrein-like peptidase 2 [hK2] in addition to age, DRE and prior biopsy status). Both tests are intended to reduce the number of unnecessary prostate biopsies in PSA-tested men. A few prospective multi-centre studies demonstrated that both the PHI and 4K score test out-performed f/t PSA PCa detection, with an improved prediction of csPCa in men with a PSA between 2–10 ng/mL [202-205]. In a head-to-head comparison both tests performed equally [206].
In contrast to the 4K score and PHI, which focus on the concentration of PSA isoforms, IsoPSA utilises a novel technology which focuses on the structure of PSA [207]. Using an aqueous two-phase solution, it partitions the isoforms of PSA and assesses for structural changes in PSA. In a multi-centre prospective validation in 271 men the assay AUC was 0.784 for high-grade vs. low-grade cancer/benign histology, which was superior to the AUCs of total PSA and percent free PSA [208]. In men with a negative mpMRI, PSA-D, 4K score and family history predicted the risk of csPCa on biopsy and using a nomogram reduced the number of negative biopsies and indolent cancers by 47% and 15%, respectively, while missing 10% of csPCas [209].
The Stockholm3 test is a prediction model that is based on several clinical variables (age, first-degree family history of PCa, and previous biopsy), blood biomarkers (total PSA, free PSA, ratio of free PSA to total PSA, human kallikrein 2, macrophage inhibitory cytokine-1, and microseminoprotein-β ), and a polygenic risk score for predicting the risk of PCa with ISUP > 2, and was shown to reduce the percent of clinically insignificant cancers when used in combination with MRI in a PSA screening population [268].
The Proclarix® test is a blood-based test that estimates the likelihood of csPCa according to measurement results for thrombospondin-1, cathepsin D, total PSA, percentage free PSA and patient age. This test has been correlated with the detection of significant PCa, notably in case of equivocal MRI (PI-RADS 3 lesions) [210].
5.2.3.2. Urine biomarkers: PCA3/SelectMDX/Mi Prostate score (MiPS)/ExoDX
Prostate cancer gene 3 (PCA3) is an overexpressed long non-coding RNA (lncRNA) biomarker that is detectable in urine sediments obtained after three strokes of prostatic massage during DRE. However, the clinical utility of the commercially available Progensa urine test for PCA3 for biopsy decision-making remains uncertain. However, combining MRI findings with the PCA3 score may improve risk stratification [211].
The SelectMDX test is similarly based on mRNA biomarker isolation from urine. The presence of HOXC6 and DLX1 mRNA levels is assessed to provide an estimate of the risk of both presence of PCa on biopsy as well as presence of high-risk cancer [212]. A multi-centre trial evaluated SelectMDX in men with a MRI PI-RADS score < 4 or PI-RADS score < 3, and the percentage of missed csPCas was 6.5% and 3.2%, respectively, whereas 45.8% and 40% of biopsies were avoided [213]. Hendriks et al., found more biopsies were avoided and more high-grade PCas detected in a MRI-based biopsy strategy compared to a SelectMDX strategy. When both tests were combined, more Gleason grade > 1 lesions were found, but the number of negative or low-grade cancer biopsies more than doubled [191]. Combining SelectMDX and MRI in men with a PSA between 3–10 ng/mL had a negative predictive value (NPV) of 93% [214]. The clinically added value of SelectMDX in the era of upfront MRI and targeted biopsies remains unclear [215].
TMPRSS2-ERG fusion, a fusion of the trans-membrane protease serine 2 (TMPRSS2) and the ERG gene can be detected in 50% of PCas [216]. When detection of TMPRSS2-ERG in urine was added to PCA3 expression and serum PSA (Mi(chigan)Prostate Score [MiPS]), cancer prediction improved [217]. Exosomes secreted by cancer cells may contain mRNA diagnostic for high-grade PCa [218,219]. Use of the ExoDx Prostate IntelliScore urine exosome assay resulted in avoiding 27% of unnecessary biopsies when compared to standard of care (SOC). However, currently, both the MiPS-score and ExoDx assay are considered investigational.
In the screening population of the ERSPC study the use of both PCA3 and 4K panel when added to the risk calculator led to an improvement in AUC of less than 0.03 [220]. Based on the available evidence, some biomarkers could help in discriminating between aggressive and non-aggressive tumours with an additional value compared to the prognostic parameters currently used by clinicians [221]. However, upfront MRI is also likely to affect the utility of above-mentioned biomarkers (see Section 5.2.3.2).
5.2.3.3. Biomarkers to select men for a repeat biopsy
In men with an elevated risk of PCa with a prior negative biopsy, the role of PHI, Progensa PCA3, and SelectMDX in deciding whether to take a repeat biopsy in men who had a previous negative biopsy is uncertain and probably not cost-effective [222]. The ConfirmMDx test is based on the concept that benign prostatic tissue in the vicinity of a PCa focus shows distinct epigenetic alterations. In case PCa is missed at biopsy, demonstration of epigenetic changes in the benign tissue would indicate the presence of carcinoma. The ConfirmMDX test quantifies the methylation level of promoter regions of three genes (Methylated APC, RASSF1 and GSTP1) in benign prostatic tissue. A multicentre study found a NPV of 88% when methylation was absent in all three markers, implying that a repeat biopsy could be avoided in these men [223]. Given the limited available data and the fact that the role of MRI in tumour detection was not accounted for, no recommendation can be made regarding the routine application of ConfirmMDX, in particular in the light of current use of MRI before biopsy.
5.2.4. Imaging
5.2.4.1. Transrectal ultrasound and ultrasound-based techniques
Standard TRUS is not reliable at detecting PCa [224] and the diagnostic yield of additional biopsies performed on hypoechoic lesions is negligible [124]. New sonographic modalities such as micro-Doppler, sonoelastography or contrast-enhanced US provided promising preliminary findings, either alone, or combined into the so-called ‘multiparametric US’ [225,226]. In the multiparametric US vs. multiparametric MRI to diagnose PCa (CADMUS) trial, 306 patients underwent both multiparametric MRI and multiparametric US composed of B-mode, Colour Doppler, real-time elastography, and contrast-enhanced US. Patients with at least one positive test underwent targeted biopsy. Multiparametric US detected 4.3% fewer csPCa while submitting 11.1% more patients to biopsy than MRI [227].
High-resolution micro-US operates at 29 MHz instead of 8-12 MHz with conventional TRUS. As high frequency ultrasonic waves are rapidly attenuated, micro-US is intrinsically limited for the exploration of the anterior part of large prostates. Several studies combined into a meta-analysis [228] obtained similar csPCa detection rates for micro-US-targeted and MRI-targeted biopsy. However, this conclusion is limited by the retrospective non-randomized design of the included studies, and by the fact that the micro-US operator was not blinded to MRI results in most of them. One prospective trial included 203 patients referred for biopsy in three centers [229]. The biopsy operator was blinded to the MRI report until after the micro-US targets had been assessed. After unblinding, micro-US and mpMRI targets were sampled, followed by systematic biopsy. Micro-US and mpMRI detected respectively 58 (73%) and 60 (76%) of the 79 csPCas, while systematic sampling detected 45/79 cases (57%). MRI-targeted biopsy detected 7 csPCas missed by micro-US; of these three were anterior lesions. Micro-US-guided biopsy detected 5 csPCas missed by MRI; of these, three were at the apex. Although these findings require further confirmation, they suggest that micro-US and MRI could complement each other. Micro-US could also be an interesting alternative to MRI/US fusion since most MRI lesions seem visible on micro-US [230]. Of note, evaluation of micro-US inter-operator variability is currently lacking.
5.2.4.2. Magnetic resonance imaging
5.2.4.2.1. MRI diagnostic performance in detecting PCa
Correlation with RP specimens shows that MRI has good sensitivity for the detection and localisation of ISUP grade > 2 cancers, especially when their diameter is larger than 10 mm [231-233]. This good sensitivity was further confirmed in patients who underwent template biopsies. In a Cochrane meta-analysis which compared MRI to template biopsies (> 20 cores) in biopsy-naive and repeat-biopsy settings, MRI had a pooled sensitivity of 0.91 (95% CI: 0.83–0.95) and a pooled specificity of 0.37 (95% CI: 0.29–0.46) for ISUP grade > 2 cancers [168]. For ISUP grade > 3 cancers, MRI pooled sensitivity and specificity were 0.95 (95% CI: 0.87–0.99) and 0.35 (95% CI: 0.26–0.46), respectively. MRI is less sensitive in identifying ISUP grade 1 PCa. It identifies less than 30% of ISUP grade 1 cancers smaller than 0.5 cc identified on RP specimens by histopathology analysis [231].
In a meta-analysis of 17 studies involving men with suspected or biopsy-proven PCa, the average PPVs for ISUP grade > 2 cancers of lesions with a PI-RADSv2.1 score of 3, 4 and 5 were 16% (7–27%), 59% (39–78%), and 85% (73–94%), respectively, but with significant heterogeneity among studies [234].
5.2.4.2.2. Targetted biopsy improves the detection of ISUP grade > 2 cancer as compared to systemic biopsy.
In pooled data of 25 reports on agreement analysis (head-to-head comparisons) between systematic biopsy (median number of cores: 8–15) and MRI-targeted biopsies (median number of cores: 2–7), the detection ratio (i.e. the ratio of the detection rates obtained by MRI-targeted biopsy alone and by systematic biopsy alone) was 1.12 (95% CI: 1.02–1.23) for ISUP grade > 2 cancers and 1.20 (95% CI: 1.06–1.36) for ISUP grade > 3 cancers, and therefore in favour of MRI-targeted biopsy [168].
Another meta-analysis of studies limited to biopsy-naïve patients with a positive MRI found that MRI-targeted biopsy detected significantly more ISUP grade > 2 cancers than systematic biopsy (risk difference, -0.11 [95% CI: -0.2 to 0.0]; p = 0.05), in prospective cohort studies (risk difference, -0.18 [95% CI: -0.24 to -0.11]; p < 0.00001), and in retrospective cohort studies (risk difference, -0.07 [95% CI: -0.12 to -0.02]; p = 0.004) [235]. This data was confirmed in prospective multi-centre trials evaluated MRI-targeted biopsy in biopsy-naïve patients [123-125].
The Target Biopsy Techniques Based on Magnetic Resonance Imaging in the Diagnosis of Prostate Cancer in Patients with Prior Negative Biopsies (FUTURE) randomised trial compared three techniques of MRI-targeted biopsy in the repeat-biopsy setting [236]. In the subgroup of 152 patients who underwent both MRI-targeted biopsy and systematic biopsy, MRI-targeted biopsy detected significantly more ISUP grade > 2 cancers than systematic biopsy (34% vs. 16%; p < 0.001, detection ratio of 2.1), which is a finding consistent with the Cochrane agreement analysis (detection ratio: 1.44). An ISUP grade > 2 cancer would have been missed in only 1.3% (2/152) of patients, had systematic biopsy been omitted [237]. These findings support that MRI-targeted biopsy significantly out-performs systematic biopsy for the detection of ISUP grade > 2 in the repeat-biopsy setting. In biopsy-naive patients, the difference appears to be less marked but remains in favour of MRI-targeted biopsy.
5.2.4.2.3. Reduced detection of ISUP grade 1 cancers by MRI-targeted biopsy without systematic biopsy
In pooled data of 25 head-to-head comparisons between systematic biopsy and MRI-targeted biopsy, the detection ratio for ISUP grade 1 cancers was 0.62 (95% CI: 0.44–0.88) in patients with prior negative biopsy and 0.63 (95% CI: 0.54–0.74) in biopsy-naive patients [168]. In the PRECISION and 4M trials, the detection rate of ISUP grade 1 patients was significantly lower in the MRI-targeted biopsy group as compared to systematic biopsy (9% vs. 22%, p < 0.001, detection ratio of 0.41 for PRECISION; 14% vs. 25%, p < 0.001, detection ratio of 0.56 for 4M) [123,125]. In the MRI-FIRST trial, MRI-targeted biopsy detected significantly fewer patients with clinically insignificant PCa (defined as ISUP grade 1 and maximum cancer core length < 6 mm) than systematic biopsy (5.6% vs. 19.5%, p < 0.0001, detection ratio of 0.29) [124]. Consequently, MRI-targeted biopsy without systematic biopsy significantly reduces over-diagnosis of low-risk disease, as compared to systematic biopsy. This seems true even when systematic biopsies are indicated after after risk stratification with a US-based risk calculator (i.e. Rotterdam Prostate Cancer Risk Calculator) [169].
5.2.4.2.4. Added value of systematic biopsy and targeted biopsy
MRI-targeted biopsies can be used in two different diagnostic pathways: 1) the ‘combined pathway’, in which patients with a positive MRI undergo combined systematic and targeted biopsy, and patients with a negative MRI undergo systematic biopsy; or 2) the ‘MRI pathway’, in which patients with a positive MRI undergo only MRI-targeted biopsy, and patients with a negative MRI who are not biopsied at all. The first diagnostic pathway focuses on maximizing the detection of significant cancers, also termed as the ‘rule-in’ ability. However, this pathway has the disadvantage of leading to a greater detection of insignificant cancers and of referring all patients with a clinical suspicion of cancer to biopsy. The second diagnostic pathway focuses on the ‘rule-out’ ability, and minimizes these disadvantages at the cost of missing a small proportion of significant cancers. Risk profiling is necessary to balance the ‘rule-in’ or ‘rule-out’ ability on an individual basis, incorporating patient and physician preference (i.e. biopsy averse/cancer averse), choosing for either adding or avoiding systematic biopsies. Table 5.3 shows the added value of systematic and MRI-targeted biopsy for ISUP grade > 2 and > 3 cancer detection.
Table 5.3: Absolute added values of targeted and systematic biopsies for ISUP grade > 2 and > 3 Cancer Detection
ISUP > 2 | ISUP > 3 | ||||||
ISUP grade | Cochrane meta-analysis* [168] | MRI-FIRST trial* [124] | 4M trial [125] | Cochrane meta-analysis* [168] | MRI-FIRST trial* [124] | 4M trial [125] | |
Biopsy-naïve | Added value of MRI-TBx | 6.3% (4.8–8.2) | 7.6% (4.6–11.6) | 7.0% (ND) | 4.7% (3.5–6.3) | 6.0% (3.4–9.7) | 3.2% (ND) |
Added value of systematic biopsy | 4.3% (2.6–6.9) | 5.2% (2.8–8.7) | 5.0% (ND) | 2.8% (1.7–4.8) | 1.2% (0.2–3.5) | 4.1% (ND) | |
Overall prevalence | 27.7% (23.7–32.6) | 37.5% (31.4–43.8) | 30% (ND) | 15.5% (12.6–19.5) | 21.1% (16.2–26.7) | 15% (ND) | |
Prior negative biopsy | Added value of MRI-TBx | 9.6% (7.7–11.8) | - | - | 6.3% (5.2–7.7) | - | - |
Added value of | 2.3% (1.2–4.5) | - | - | 1.1% (0.5–2.6) | - | - | |
Overall prevalence | 22.8% (20.0–26.2) | - | - | 12.6% (10.5–15.6) | - | - | |
*Intervals in parenthesis are 95% CI.
The absolute added value of a given biopsy technique is defined by the percentage of patients of the entire cohort diagnosed only by this biopsy technique.
ISUP = International Society of Urological Pathology (grade); MRI-TBx = magnetic resonance imaging-targeted biopsies; ND = not defined.
In Table 5.3, the absolute added values refer to the percentage of patients in the entire cohort; if the cancer prevalence is taken into account, the ‘relative’ percentage of additional detected PCa can be computed. Adding MRI-targeted biopsy to systematic biopsy in biopsy-naïve patients increases the number of detected ISUP grade > 2 and grade > 3 PCa by approximately 20% and 30%, respectively. In the repeat-biopsy setting, adding MRI-targeted biopsy increases detection of ISUP grade > 2 and grade > 3 PCa by approximately 40% and 50%, respectively. Omitting systematic biopsy in biopsy-naïve patients would miss approximately 16% of all detected ISUP grade > 2 PCa and 18% of all ISUP grade > 3 PCa. In the repeat-biopsy setting, it would miss approximately 10% of ISUP grade > 2 PCa and 9% of ISUP grade > 3 PCa.
In the GÖTEBORG-2 prospective trial, 37,887 men between 50 and 60 years of age were invited to undergo regular PSA screening [238]. Participants with a PSA level above 3 ng/mL were randomly allocated to MRI and combined systematic- and targeted biopsy (reference group) or to MRI and targeted biopsy only in case of PI-RADS > 3 lesions (experimental group). In the experimental group, the detection rate of ISUP 1 cancers was reduced by half (detection ratio: 0.46, 95% CI: 0.33–0.64, p < 0.001); that of ISUP > 2 cancers was lower but not significantly (detection ratio: 0.81, 95% CI: 0.60 to 1.1). In the reference group, 10 of the 68 men with ISUP > 2 cancer were diagnosed by systematic biopsy only. All these 10 patients were of intermediate risk. Thus, in a screening setting, the ‘MRI pathway’ may reduce the risk of over-diagnosis by half, at the cost of delaying detection of intermediate-risk tumours in a small percentage of patients. However, these good results were obtained at a single academic centre with double reading of the MRI, which may limit their generalisability in less experienced centers (see Sections 5.2.4.2.6.1 and 5.2.4.2.6.2).
5.2.4.2.5. Avoiding biopsies in the ‘MR pathway’
The diagnostic yield and number of biopsy procedures potentially avoided by the ‘MR pathway’ depends on the Likert/PI-RADS threshold used to define a positive MRI. In pooled studies on biopsy-naïve patients and patients with prior negative biopsies, a Likert/PI-RADS threshold of > 3 would have avoided 30% (95% CI: 23–38) of all biopsy procedures while missing 11% (95% CI: 6–18) of all detected ISUP grade > 2 cancers (relative percentage) [168]. Increasing the threshold to > 4 would have avoided 59% (95% CI: 43–78) of all biopsy procedures while missing 28% (95% CI: 14–48) of all detected ISUP grade > 2 cancers [168]. Of note, the percentages of negative MRI (Likert/PI-RADS score < 2) in the MRI-FIRST, PRECISION and 4M trials were 21.1%, 28.9% and 49%, with related ISUP grade > 2 cancer prevalence of 27.7% (23.7–32.6), 37.5% (31.4–43.8), and 30% (ND) respectively [123-125].
5.2.4.2.6. Practical considerations
5.2.4.2.6.1. Prostate MRI reproducibility
Despite the use of the PI-RADSv2 scoring system [239], MRI inter-reader reproducibility remains moderate at best which currently limits its broad use by non-dedicated radiologists [240]. However, significant improvement in the accuracy of MRI and MRI-targeted biopsy can be observed over time, both in academic and community hospitals, especially after implementation of PI-RADSv2 scoring and multidisciplinary meetings using pathological correlation and feedback [241-244]. An updated version of the PI-RADS score (PI-RADSv2.1) has been published to improve reader reproducibility, showing improved diagnostic performance [234] but it has not yet been fully evaluated [245]. It is still too early to predict whether quantitative approaches and computer-aided diagnostic systems will improve the characterisation of lesions seen at MRI [246]. Standardisation of MRI interpretation and quality check of acquisition and of MRI-targeted biopsy technique is required to optimise the ‘MRI pathway’ in large-volume and small-volume (non-expert) centres [247-249].
5.2.4.2.6.2. Targeted biopsy accuracy and reproducibility
Clinically significant PCa not detected by the ‘MRI pathway’ can be missed because of MRI failure (invisible cancer or reader’s misinterpretation) or because of targeting failure (target missed or under sampled by MRI-targeted biopsy).
The accuracy of MRI-targeted biopsy is substantially impacted by the experience of the biopsy operator [240], and two retrospective studies on patients with a unilateral lesion found that the added value of systematic biopsy was higher in the MRI-positive lobe than in the MRI-negative lobe [250,251]. This suggests that a substantial part of the added value of systematic biopsies is due to mistargeting issues (i.e., the lesion has been correctly identified by MRI but missed by MRI-targeted biopsy and detected by systematic sampling).
Increasing the number of cores taken per target may partially compensate for guiding imprecision, and a minimum of 3 to 5 cores is required for proper sampling of the lesions [252-254]. Consequently, some authors have suggested MRI-directed biopsy approaches using targeted biopsies combined with peri-lesional/regional systematic biopsies, rather than standard sextant-based systematic biopsies. This could decrease the number of cores taken (by avoiding systematic biopsies in MRI-negative lobes) and improve the detection of csPCa (by improving the lesional and peri-lesional sampling). A recent meta-analysis of 8 retrospective studies showed a nonsignificant difference in detection of ISUP grade > 2 PCa in the MRI-directed targeted- and regional biopsy approach, compared to the recommended practice of MRI-directed targeted- and systematic biopsy approach (RR: 0.95, 95% CI: 0.90–1.01; p = 0.09). However MRI-directed targeted- and regional biopsy approach detected significantly more csPCa than MRI-targeted biopsy alone (RR: 1.18, 95% CI: 1.10–1.25; p < 0.001) [255]. However, due to the heterogeneity of the retrospective studies, prospective clinical studies are needed before the role of this biopsy approach can be assessed. The impact of per-lesional sampling on non-significant (ISUP 1) cancer has not been fully assessed either.
5.2.4.2.6.3. Risk-stratification
Using risk-stratification to avoid biopsy procedures
Prostate-specific antigen density may help refine the risk of csPCa in patients undergoing MRI as PSA-D and the PI-RADS score are significant independent predictors of csPCa at biopsy [256,257]. In a meta-analysis of 8 studies, pooled MRI NPV for ISUP grade > 2 cancer was 84.4% (95% CI: 81.3–87.2) in the whole cohort, 82.7% (95% CI: 80.5–84.7) in biopsy-naive men and 88.2% (95% CI: 85–91.1) in men with prior negative biopsies. In the subgroup of patients with PSA-D < 0.15 ng/mL, NPV increased to respectively 90.4% (95% CI: 86.8–93.4), 88.7% (95% CI: 83.1–93.3) and 94.1% (95% CI: 90.9–96.6) [258]. In contrast, the risk of csPCa is as high as 27–40% in patients with negative MRI and PSA-D > 0.15–0.20 ng/mL/cc [125,172,257,259-261].
Based on a meta-analysis of > 3,000 biopsy-naïve men, a risk-adapted data table of csPCa was developed, linking PI-RADS score (1-2, 3, and 4-5) to PSA-D categories (< 0.10, 0.10–0.15, 0.15–0.20 and > 0.20 ng/mL) (Table 5.4) [170]. For example, the risk of having ISUP grade > 2 cancer in biopsy-naïve men with a PI-RADS 1–2 assessment score and PSA-D below 0.10 is 3–4%, in a below-average-risk population of < 5% [170]. This risk-adapted matrix table based on PSA-D and on MRI risk assessments may guide the decision to perform a biopsy.
These data are applicable for a mean ISUP grade > 2 cancer prevalence of 35% (range 28–46%) in biopsy-naïve men, and would need to be adjusted to other populations’ prevalence. Awaiting validation of MRI-based multivariate risk-prediction tools, corroboration linking MRI findings to PSA-D values for biopsy decisions is beginning to emerge which may promote their routine use in clinical practice [20,262]. It must be emphasised, however, that the use of PSA-D remains currently limited due to the lack of standardisation of prostate volume measurement (assessed by DRE or by imaging [TRUS or MRI using various techniques such as ellipsoid formula or planimetry]). The impact of this lack of standardisation on the volume estimation remains under evaluated.
Table 5.4: Risk data table of clinically significant prostate cancer, related to PI-RADS score and PSA-D categories in biopsy-naïve men, clinically suspected of having significant disease [170] *
Detection of clinically significant prostate cancer (ISUP grade 2 and higher) | |||||
PSA-density risk groups | |||||
PI-RADS risk categories | Prevalence ISUP > 2 PCa | Low < 0.10 | Intermediate-low 0.10–015 | Intermediate-high 0.15–0.20 | High > 0.20 |
31% (678/2199) | 28% (612/2199) | 16% (360/2199) | 25% (553/2199) | ||
Compiled totals of csPCa risk | |||||
PI-RADS 1–2 | 6% (48/839) | 3% (11/411) | 7% (17/256) | 8% (8/104) | 18% (12/68) |
PI-RADS 3 | 16% (41/254) | 4% (3/74) | 13% (11/88) | 29% (12/41) | 29% (15/51) |
PI-RADS 4–5 | 62% (687/1106) | 31% (59/189) | 54% (144/286) | 69% (148/215) | 77% (336/434) |
All PI-RADS | 35% (776/2199) | 11% (73/674) | 28% (172/612) | 47% (168/360) | 66% (363/553) |
Risk-adapted matrix table for biopsy decision management | |||||
PI-RADS 1–2 | No biopsy | No biopsy | No biopsy | Consider biopsy | |
PI-RADS 3 | No biopsy | Consider biopsy | Highly consider biopsy | Perform biopsy | |
PI-RADS 4–5 | Perform biopsy | Perform biopsy | Perform biopsy | Perform biopsy | |
very low | 0–5% csPCa (below population risk) # |
low | 5–10% csPCa (acceptable risk) ## |
Intermediate-low | 10–20% csPCa |
Intermediate-high | 20–30% csPCa |
High | 30–40% csPCa |
Very high | > 40% csPCa |
# Thompson IM et al. N Engl J Med. 2004 May 27;350(22):2239-46. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng/mL.
## 2019 EAU guidelines: csPCa 9% (95% CI: 6–14%).
Table adapted from: Schoots, IG and Padhani AR. BJU Int 2021 127(2):175. Risk-adapted biopsy decision based on prostate magnetic resonance imaging and prostate-specific antigen density for enhanced biopsy avoidance in first prostate cancer diagnostic evaluation, with permission from Wiley.
Several groups have developed comprehensive risk calculators which combine MRI findings with simple clinical data as a tool to predict subsequent biopsy results [263]. At external validation, they tended to outperform risk calculators not incorporating MRI findings (ERSPC and Prostate Cancer Prevention Trial) with good discriminative power (as measured by the AUC). However, they also tended to be miscalibrated with under- or over-prediction of the risk of ISUP grade > 2 cancer [264,265]. In one study that externally assessed four risk calculators combining MRI findings and clinical data, only two demonstrated a distinct net benefit when a risk of false-negative prediction of 15% was accepted. The others were harmful for this risk level, as compared to the ‘biopsy all’ strategy [264]. This illustrates the prevalence-dependence of risk models. Recalibrations taking into account the local prevalence are possible, but this approach is difficult in routine clinical practice as the local prevalence is difficult to estimate and may change over time.
Using risk-stratification to avoid MRI scans and biopsy procedures
A retrospective analysis including 200 men from a prospective database of patients who underwent MRI and combined systematic and targeted biopsy showed that upfront use of the Rotterdam Prostate Cancer Risk Calculator would have avoided MRI and biopsy in 73 men (37%). Of these 73 men, 10 had ISUP grade 1 cancer and 4 had ISUP grade > 2 cancer [266]. A prospective multi-centre study evaluated several diagnostic pathways in 545 biopsy-naive men who underwent MRI and systematic and targeted biopsy. Using a PHI threshold of > 30 to perform MRI and biopsy would have avoided MRI and biopsy in 25% of men at the cost of missing 8% of the ISUP grade > 2 cancers [267]. Another prospective multi-centre trial including 532 men (with or without history of prostate biopsy) showed that using a threshold of > 10% for the Stockholm3 test to perform MRI and biopsy would have avoided MRI and biopsy in 38% of men at the cost of missing 8% of ISUP grade > 2 cancers [268].
5.2.4.2.6.4. Potential cancer grade shift, induced by improved diagnosis by MRI and MRI-targeted biopsy
MRI findings are significant predictors of adverse pathology features on prostatectomy specimens, and of survival-free BCR after RP or RT [105,269-271]. In addition, tumours visible on MRI are enriched in molecular hallmarks of aggressivity, as compared to invisible lesions [272]. Thus, MRI does identify aggressive tumours.
Nonetheless, as MRI-targeted biopsy is more sensitive than systematic biopsy in detecting areas of high-grade cancer, ISUP grade > 2 cancers detected by MRI-targeted biopsy are, on average, of better prognosis than those detected by the classical diagnostic pathway (Will Rogers phenomenon [107]). This is illustrated in a retrospective series of 1,345 patients treated by RP which showed that, in all risk groups, patients diagnosed by MRI-targeted biopsy had better BCR-free survival than those diagnosed by systematic biopsy only [105]. To mitigate this grade shift, in case of targeted biopsies, the 2019 ISUP consensus conference recommended using an aggregated ISUP grade summarizing the results of all biopsy cores from the same MR lesion, rather than using the result from the core with the highest ISUP grade [110]. When long-term follow-up of patients who underwent MRI-targeted biopsy is available, a revision of the risk-groups definition will become necessary. In the meantime, results of MRI-targeted biopsy must be interpreted in the context of this potential grade shift [273].
5.2.4.3. Guidelines for MRI imaging in biopsy decision
Recommendations for all patients | Strength rating |
Do not use magnetic resonance imaging (MRI) as an initial screening tool. | Strong |
Adhere to PI-RADS guidelines for MRI acquisition and interpretation and evaluate MRI results in multidisciplinary meetings with pathological feedback. | Strong |
Recommendations in biopsy-naïve patients | Strength rating |
Perform MRI before prostate biopsy. | Strong |
When MRI is positive (i.e., PI-RADS > 3), combine targeted and systematic biopsy. | Strong |
When MRI is negative (i.e., PI-RADS < 2), and clinical suspicion of PCa is low (e.g., PSA density < 0.15 ng/mL), omit biopsy based on shared decision-making with the patient. | Weak |
Recommendations in patients with prior negative biopsy | Strength rating |
Perform MRI before prostate biopsy. | Strong |
When MRI is positive (i.e., PI-RADS > 3), perform targeted biopsy only. | Weak |
When MRI is negative (i.e., PI-RADS < 2), and clinical suspicion of PCa is high, perform systematic biopsy based on shared decision-making with the patient. | Strong |
5.2.5. Baseline biopsy decision
The need for prostate biopsy is based on PSA level, PSA density, other biomarkers and/or suspicious DRE and/or imaging (see Section 5.2.4). Age, potential co-morbidity and therapeutic consequences should also be considered and discussed beforehand [241]. Risk stratification is a potential tool for reducing unnecessary biopsies [274].
Limited PSA elevation alone should not prompt immediate biopsy. Prostate-specific antigen level should be verified after a few weeks, in the same laboratory using the same assay under standardised conditions (i.e. no ejaculation, manipulations, and urinary tract infections [UTIs]) [275,276]. Empiric use of antibiotics in an asymptomatic patient in order to lower the PSA should not be undertaken [277].
Ultrasound (US)-guided and/or MRI-targeted biopsy is now the SOC. Prostate biopsy is performed by either the recommended transperineal approach or the transrectal one. Cancer detection rates, when performed without prior imaging with MRI, are comparable between the two approaches [246], however, evidence suggests reduced infection risk with the transperineal route (see Section 5.2.8.1.1) [278,279]. Transurethral resection of the prostate (TURP) should not be used as a tool for cancer detection [280].
5.2.6. Repeat biopsy decision
5.2.6.1. Repeat biopsy after previously negative biopsy
Men with a previous negative systematic biopsy should be offered a prostate MRI and in case of PI-RADS > 3 findings, a repeat (targeted) biopsy should be done. Other indications for repeat biopsy should be discussed on an individual basis taking into account PSA level and its evolution, DRE, PSA density and pathological findings on the previous biopsy.
In a contemporary series of biopsies the likelihood of finding a csPCa after follow-up biopsy after a diagnosis of atypical small acinar proliferation and high-grade prostatic intraepithelial neoplasia (PIN) was only 6-8%, not significantly different from follow-up biopsies after a negative biopsy [281,282].
The added value of other biomarkers remains unclear (see Sections 5.2.3.1 and 5.2.3.2).
5.2.6.2. Saturation biopsy
The incidence of PCa detected by saturation repeat biopsy (> 20 cores) is 30–43% and depends on the number of cores sampled during earlier biopsies [283]. Saturation biopsy may be performed with the transperineal technique, which detects an additional 38% of PCa. The rate of urinary retention varies substantially from 1.2% to 10% [284-287].
However, given the very low risk of subsequent csPCa after a negative biopsy and/or in case of negative MRI, the clinical utility of saturation biopsy in the repeat biopsy setting remains uncertain in the current MRI-driven diagnostic pathway and such schemes should not be routinely used [288].
5.2.7. Prostate biopsy procedure
For systematic biopsies, where no prior imaging is used for targeting, the sample sites should be bilateral from apex to base, as far posterior and lateral as possible in the peripheral gland regardless of the approach used. A 2006 systematic review showed that 12 is the minimum number of cores for systematic biopsies, with > 12 cores not increasing cancer detection rate significantly [289].
Additional cores should be obtained from suspect areas identified by DRE or on pre-biopsy MRI; multiple cores (3–5) should be taken from each MRI-visible lesion.
Where MRI has shown a suspicious lesion, MR-targeted biopsy can be obtained through cognitive guidance, US/MR fusion software or direct in-bore guidance. Current literature, including systematic reviews and
meta-analyses, does not show a clear superiority of one image-guided technique over another [236,290-293].
However, regarding approach, the only systematic review and meta-analysis comparing MRI-targeted transrectal biopsy to MRI-targeted transperineal biopsy, analysing 8 studies, showed a higher sensitivity for detection of csPCa when the transperineal approach was used (86% vs. 73%) [294]. This benefit was especially pronounced for anterior tumours. Multiple cores (3–5) should be taken from each lesion (see Section 5.2.4.2.6.2).
As detailed in Section 5.2.4.2.6.2, the added value of systematic biopsy is partially explained by the fact that they compensate for guiding imprecisions of targeted biopsy. Therefore, biopsy strategies with multiple
peri-lesional (regional) targeted cores obtained in addition of MRI-directed targeted cores are being investigated [250,251,295-298]. Prospective clinical trials are needed to evaluate whether these strategies can replace the combination of systematic and targeted biopsy currently recommended as the diagnostic work-up in men with positive MRI scans.
5.2.8. Summary of evidence and guidelines for prostate biopsies
Summary of evidence | LE |
Literature review including multiple biopsy schemes suggests that a minimum 12-core scheme is optimal in the majority of initial and repeat biopsy patients, dependent on prostate size. These biopsy schemes should be heavily weighted towards the lateral aspect and the apex of the prostate to maximize peripheral zone sampling [299]. | 3 |
Systematic review and meta-analysis comparing MRI-targeted transrectal biopsy to MRI-targeted transperineal biopsy, analysing 8 studies, showed a higher sensitivity for detection of csPCa when the transperineal approach was used (86% vs. 73%). | 2 |
Current literature, including systematic reviews and meta-analyses, does not show a clear superiority of one image-guided technique (cognitive guidance, US/MR fusion software or direct in-bore guidance) over the other. | 2 |
Recommendations | Strength rating |
When performing systematic biopsy only, at least 12 cores are recommended. | Strong |
Systematic transperineal biopsies are preferred over systematic transrectal biopsies for detection of clinically significant PCa. | Strong |
Where magnetic resonance imaging (MRI) has shown a suspicious lesion, MR-targeted biopsy can be obtained through cognitive guidance, US/MR fusion software or direct in-bore guidance. | Weak |
5.2.8.1. Antibiotics prior to biopsy
5.2.8.1.1. Transperineal prostate biopsy
A total of eight randomised studies including 1,596 patients compared the impact of biopsy route on infectious complications. Infectious complications were significantly higher following transrectal biopsy (48 events among 789 men) compared to transperineal biopsy (22 events among 807 men) (RR: 95% CI: 2.48 [1.47–4.2]) [300,301]. In addition, a systematic review including 165 studies with 162,577 patients described sepsis rates of 0.1% and 0.9% for transperineal and transrectal biopsies, respectively [302]. Finally, a population-based study from the UK (n = 73,630) showed lower re-admission rates for sepsis in patients who had transperineal vs. transrectal biopsies (1.0% vs. 1.4%, respectively) [303]. The available evidence demonstrates that the transrectal approach should be abandoned in favour of the transperineal approach despite any possible logistical challenges. A systematic review and meta-analysis of eight non-RCTs reported no significant differences between patients receiving or not receiving antibiotic prophylaxis in terms of post-biopsy infection (0,11% vs. 0.31%) and sepsis (0.13% vs. 0,09%), for the transperineal approach [304]. This is in line with another systematic review and meta-analysis of 112 individual patient cohorts which also showed no significant difference in the number of patients experiencing post-transperineal-biopsy infection 1.35% of 29,880 patients receiving antibiotic prophylaxis and 1.22% of 4,772 men not receiving antibiotic prophylaxis (p = 0.8) [305]. In addition, two recently published RCTs have reported comparably low post-biopsy infection rates for transperineal biopsy regardless of whether antibiotic prophylaxis was administered or not [306,307].
There is a growing body of evidence to suggest that antibiotic prophylaxis may not be required for transperineal biopsy; however the Panel has chosen to wait until a number of ongoing RCTs report their study findings before making a recommendation on this.
5.2.8.1.2. Transrectal prostate biopsy
An updated meta-analysis of eleven RCTs including 2,237 men showed that use of a rectal povidone-iodine preparation before biopsy, in addition to antimicrobial prophylaxis, resulted in a significantly lower rate of infectious complications (RR: 95% CI: 0.47 [0.36–0.61]) [301,308-310]. Single RCTs showed no evidence of benefit for perineal skin disinfection [311], but reported an advantage for rectal povidone-iodine preparation before biopsy compared to after biopsy [312].
A meta-analysis of four RCTs including 671 men evaluated the use of rectal preparation by enema before transrectal biopsy. No significant advantage was found regarding infectious complications (RR: 95% CI: 0.96 [0.64–1.54]) [301].
An updated meta-analysis of 28 RCTs with 4,027 patients found no evidence that use of peri-prostatic injection of local anaesthesia resulted in more infectious complications than no injection (RR: 95% CI: 1.08 [0.79–1.48]) [300,301,313]. A meta-analysis of 9 RCTs including 2,230 patients found that extended biopsy templates showed comparable infectious complications to standard templates (RR: 95% CI: 0.80 [0.53–1.22]) [301]. Additional meta-analyses found no difference in infectious complications regarding needle guide type (disposable vs. reusable), needle type (coaxial vs. non-coaxial), needle size (large vs. small), and number of injections for peri-prostatic nerve block (standard vs. extended) [301].
A meta-analysis of eleven studies with 1,753 patients showed significantly reduced infections after transrectal prostate biopsy when using antimicrobial prophylaxis as compared to placebo/control (RR: 95% CI: 0.56 [0.40–0.77]) [314].
Fluoroquinolones have been traditionally used for antibiotic prophylaxis in this setting; however, overuse and misuse of fluoroquinolones has resulted in an increase in fluoroquinolone resistance. In addition, the European Commission has implemented stringent regulatory conditions regarding the use of fluoroquinolones resulting in the suspension of the indication for peri-operative antibiotic prophylaxis including prostate biopsy [315].
A systematic review and meta-analysis on antibiotic prophylaxis for the prevention of infectious complications following prostate biopsy concluded that in countries where fluoroquinolones are allowed as antibiotic prophylaxis, a minimum of a full one-day administration, as well as targeted therapy in case of fluoroquinolone resistance, or augmented prophylaxis (combination of two or more different classes of antibiotics) is recommended [314]. In countries where use of fluoroquinolones are suspended, cephalosporins or aminoglycosides can be used as individual agents with comparable infectious complications based on meta-analysis of two RCTs [314]. A meta-analysis of three RCTs reported that fosfomycin trometamol was superior to fluoroquinolones (RR: 95% CI: 0.49 [0.27–0.87]) [314], but routine general use should be critically assessed due to the relevant infectious complications reported in non-randomised studies [316]. Of note the indication of fosfomycin trometamol for prostate biopsy has been withdrawn in Germany as the manufacturers did not submit the necessary pharmacokinetic data in support of this indication. Urologists are advised to check their local guidance in relation to the use of fosfomycin trometamol for prostate biopsy. Another possibility is the use of augmented prophylaxis without fluoroquinolones, although no standard combination has been established to date. Finally, targeted prophylaxis based on rectal swap/stool culture is plausible, but no RCTs are available on non-fluoroquinolones. See figure 5.1 for prostate biopsy workflow to reduce infections complications.
5.2.8.2. Summary of evidence and recommendations for performing prostate biopsy (in line with the EAU Urological Infections Guidelines Panel)
Summary of evidence | LE |
A meta-analysis of eight studies including 1,596 patients showed significantly reduced infectious complications in patients undergoing transperineal biopsy as compared to transrectal biopsy. | 1a |
A meta-analysis of eight non-RCTS reported comparable rates of post-biopsy infections in patients undergoing transperineal biopsy irrespective if antibiotic prophylaxis was given or not. | 1a |
A meta-analysis of eleven RCTs including 2,036 men showed that use of a rectal povidone-iodine preparation before transrectal biopsy, in addition to antimicrobial prophylaxis, resulted in a significantly lower rate of infectious complications. | 1a |
A meta-analysis on eleven studies with 1,753 patients showed significantly reduced infections after transrectal biopsy when using antimicrobial prophylaxis as compared to placebo/control. | 1a |
Recommendations | Strength rating* |
Perform prostate biopsy using the transperineal approach due to the lower risk of infectious complications. | Strong |
Use routine surgical disinfection of the perineal skin for transperineal biopsy. | Strong |
Use rectal cleansing with povidone-iodine prior to transrectal prostate biopsy. | Strong |
Do not use fluoroquinolones for prostate biopsy in line with the European Commission final decision on EMEA/H/A-31/1452. | Strong |
Use either target prophylaxis based on rectal swab or stool culture; augmented prophylaxis (two or more different classes of antibiotics); or alternative antibiotics (e.g., fosfomycin trometamol**, cephalosporin, aminoglycoside) for antibiotic prophylaxis for transrectal biopsy. | Weak |
Ensure that prostate core biopsies from different sites are submitted separately for processing and pathology reporting. | Strong |
* Note on strength ratings:The above strength ratings are explained here due to the major clinical implications of these recommendations. Although data showing the lower risk of infection via the transperineal approach is low in certainty, its statistical and clinical significance warrants its Strong rating. Strong ratings are also given for routine surgical disinfection of skin in transperineal biopsy and povidone-iodine rectal cleansing in transrectal biopsy as, although quality of data is low, the clinical benefit is high and practical application simple. A ‘Strong’ rating is given for avoiding fluoroquinolones in prostate biopsy due to its legal implications in Europe.
** The indication of fosfomycin trometamol for prostate biopsy has been withdrawn in Germany as the manufacturers did not submit the necessary pharmacokinetic data in support of this indication. Urologists are advised to check their local guidance in relation to the use of fosfomycin trometamol for prostate biopsy.
Figure 5.1: Prostate biopsy workflow to reduce infectious complications*
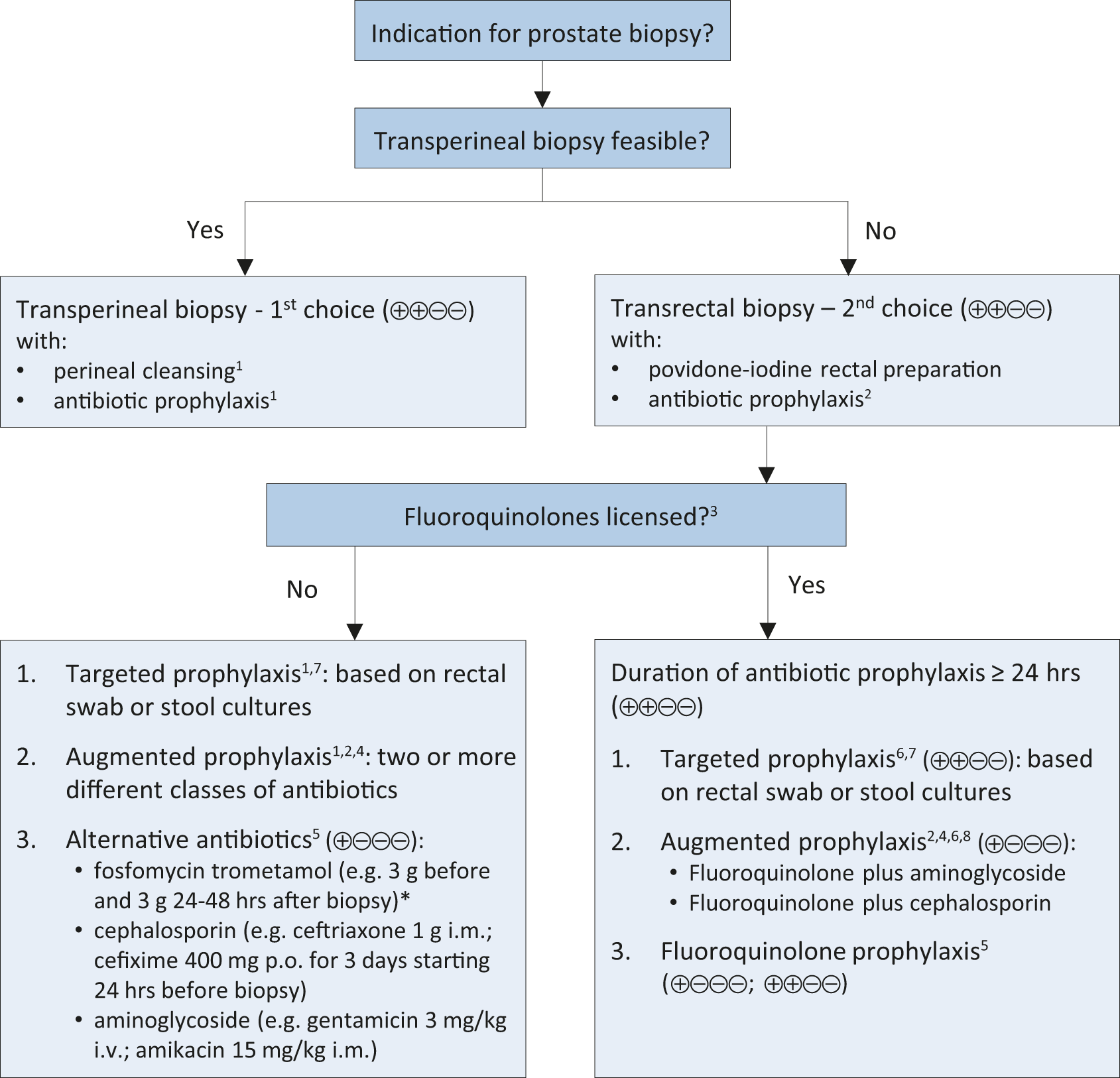
Suggested workflow on how to reduce post biopsy infections.
1. Two systematic reviews including non-RCTs and two RCTs describe comparable rates of post-transperineal biopsy infection in patients with and without antibiotic prophylaxis.
2. Be informed about local antimicrobial resistance.
3. Banned by European Commission due to side effects.
4. Contradicts principles of Antimicrobial Stewardship.
5. Fosfomycin trometamol (3 RCTs), cephalosporins (2 RCTs), aminoglycosides (2 RCTs).
6. Only one RCT comparing targeted and augmented prophylaxis.
7. Originally introduced to use alternative antibiotics in case of fluoroquinolone resistance.
8. Various schemes: fluoroquinolone plus aminoglycoside (3 RCTs); and fluoroquinolone plus cephalosporin (1 RCT).GRADE Working Group grades of evidence. High certainty: (⊕⊕⊕⊕) very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: (⊕⊕⊕⊖) moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: (⊕⊕⊖⊖) confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: (⊕⊖⊖⊖) very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. Figure adapted from Pilatz et al. [314], with permission from Elsevier.*
Of note: local guidance in relation to the use of fosfomycin trometamol for prostate biopsy needs to be checked.
5.2.8.3. Local anaesthesia prior to biopsy
Ultrasound-guided peri-prostatic block is recommended [317]. Ten mL of 2% lidocaine is infiltrated bilaterally along the apex to base. Intra-rectal instillation of local anaesthesia is inferior to peri-prostatic infiltration [318]. Local anaesthesia can also be used effectively for MRI-targeted and systematic transperineal biopsy [319]. Patients are placed in the lithotomy position. Twenty mL of 0.5% bupivacaine with adrenaline (1 in 200,000) is injected into the perineal skin and subcutaneous tissues anterior to the anus, followed two minutes later by a peri-prostatic block. A systematic review evaluating pain in 3 studies comparing transperineal vs. transrectal biopsies found that the transperineal approach significantly increased patient pain (RR: 1.83 [1.27–2.65]) [320]. In a randomised comparison a combination of peri-prostatic and pudendal block anaesthesia reduced pain during transperineal biopsies compared to peri-prostatic anaesthesia only [321]. Targeted biopsies can then be taken via a brachytherapy grid or a freehand needle-guiding device under local infiltration anaesthesia
[319,322,323].
5.2.8.4. Complications
Complications of TRUS biopsy are listed in Table 5.5 [324]. Mortality after prostate biopsy is extremely rare and most are consequences of sepsis [325]. Low-dose aspirin is no longer an absolute contra-indication [326]. A systematic review found favourable infection rates for transperineal compared to TRUS biopsies with similar rates of haematuria, haematospermia and urinary retention [327]. A meta-analysis of 4,280 men randomised between transperineal vs. TRUS biopsies in 13 studies found no significant differences in complication rates, however, data on sepsis compared only 497 men undergoing TRUS biopsy to 474 having transperineal biopsy. The transperineal approach required more (local) anaesthesia [328].
Table 5.5: Adverse events of three groups of targeted biopsy *
Overall | Transrectal | Transperineal | Transrectal | p value | |
Clavien-Dindo grade | < 0.001 | ||||
No adverse events | 30.3 (71) | 47.4 (36) | 29.1 (23) | 15.4 (12) | |
Grade 1 | 63.2 (148) | 50.0 (38) | 65.8 (52) | 74.4 (58) | |
Grade 2 | 6.0 (14) | 2.6 (2) | 5.1 (4) | 10.3 (8) | |
Grades 3, 4, 5 | – | – | – | – | |
Haematuria | 53.4 (125) | 35.5 (27) | 50.6 (40) | 74.4 (58) | < 0.001 |
Haematospermia | 37.2 (87) | 26.3 (20) | 35.4 (28) | 50.0 (39) | < 0.01 |
Rectal bleeding | 3.4 (8) | 2.6 (2) | 2.5 (2) | 5.1 (4) | 0.59 |
UTI | 3.4 (8) | 2.6 (2) | 1.3 (1) | 6.4 (5) | 0.21 |
Fever | 3 (7) | 1.3 (1) | 2.5 (2) | 5.1 (4) | 0.46 |
Urinary retention | 3 (7) | – | 3.8 (3) | 5.1 (4) | 0.15 |
Haematoma | 1.3 (3) | – | 3.8 (3) | – | 0.29 |
Other | 0.56 | ||||
Lower back pain | 0.9 (2) | 1.3 (1) | 1.3 (1) | – | |
Atrial fibrillation | 0.4 (1) | – | 1.3 (1) | – |
COG-TB = cognitive registration TRUS targeted biopsy; FUS-TB = MRI-TRUS fusion targeted biopsy; biopsy; MRI = magnetic resonance imaging; MRI-TB = in-bore MRI targeted biopsy; TB = targeted biopsy; TRUS = transrectal ultrasound; UTI = urinary tract infection. Data are presented as % (n).
*With permission by Elsevier.
5.2.8.5. Seminal vesicle biopsy
Indications for SV (staging) biopsies are poorly defined. At a PSA of > 15 ng/mL, the odds of tumour involvement are 20–25% [329]. A SV staging biopsy is only useful if it has a decisive impact on treatment, such as ruling out radical tumour resection or for potential subsequent RT. Its added value compared with MRI is questionable.
5.2.8.6. Transition zone biopsy
Transition zone sampling during baseline biopsies has a low detection rate and should be limited to MRI-detected lesions or repeat template biopsies [330].
5.2.9. Pathology of prostate needle biopsies
5.2.9.1. Processing
Prostate core biopsies from different sites are processed separately, as delivered by the biopsy operator. Before processing, the number and length of the cores are recorded. The length of biopsy tissue significantly correlates with the PCa detection rate [331]. In case individual cores can clearly be identified in submitted jars, a maximum of three cores should be embedded per tissue cassette, and sponges or paper should be used to keep the cores stretched and flat to achieve optimal flattening and alignment [332-334]. To optimise detection of small lesions and improve accuracy of grading, paraffin blocks should be cut at three levels and intervening unstained sections may be kept for immunohistochemistry (IHC) [335].
5.2.9.2. Microscopy and reporting
Diagnosis of PCa is based on histology. The diagnostic criteria include features pathognomonic of cancer, major and minor features favouring cancer and features against cancer. Ancillary staining and additional (deeper) sections should be considered if a suspect lesion is identified [335]. Diagnostic uncertainty is resolved by intradepartmental or external consultation [335]. Sections 5.2.9.2.1 and 5.2.9.2.2 list the recommended terminology and item list for reporting prostate biopsies [332]. Type and subtype of PCa should be reported such as for instance acinar adenocarcinoma, ductal adenocarcinoma and small or large cell neuroendocrine carcinoma, even if representing a small proportion of the PCa. The distinct aggressive nature of small/large cell neuroendocrine carcinoma should be commented upon in the pathology report [332]. Apart from grading acinar and ductal adenocarcinoma, the percentage of Gleason grade 4 component should be reported in Gleason score 7 (3+4 and 4+3) PCa biopsies. Percentage Gleason grade 4 has additional prognostic value and is considered in some AS protocols [336-338]. Considerable evidence has been accumulated in recent years supporting that among the Gleason grade 4 patterns, cribriform pattern carries an increased risk of biochemical recurrence, metastatic disease and death of disease [339,340]. Reporting of this sub-pattern based on established criteria is recommended [110,341]. Intraductal carcinoma, defined as an extension of cancer cells into pre-existing prostatic ducts and acini, distending them, with preservation of basal cells [110], should be distinguished from high-grade PIN [342] as it conveys unfavourable prognosis in terms of biochemical recurrence and cancer-specific survival (CSS) [343,344]. Its presence should be reported, whether occurring in isolation or associated with adenocarcinoma [110].
5.2.9.2.1. Recommended terminology for reporting prostate biopsies
Benign/negative for malignancy; if appropriate, include a description |
Active inflammation |
Granulomatous inflammation |
High-grade prostatic intraepithelial neoplasia (PIN) |
High-grade PIN with atypical glands, suspicious for adenocarcinoma |
Focus of atypical glands/lesion suspicious for adenocarcinoma/atypical small acinar proliferation, suspicious for cancer |
Adenocarcinoma, provide type and subtype, and presence or absence of cribriform pattern |
Intraductal carcinoma |
Each biopsy site should be reported individually, including its location (in accordance with the sampling site) and histopathological findings, which include the histological type and the ISUP 2019 grade [110,345,346]. For MRI targeted biopsies consisting of multiple cores per target the aggregated (or composite) ISUP grade should be reported per targeted lesion [110]. If the targeted biopsies are negative, presence of specific benign pathology should be mentioned, such as dense inflammation, fibromuscular hyperplasia or granulomatous inflammation [110,347]. A global ISUP grade comprising all systematic (non-targeted) and targeted biopsies is also reported (see Section 4.2). The global ISUP grade takes into account all biopsies positive for carcinoma, by estimating the total extent of each Gleason grade present. For instance, if three biopsy sites are entirely composed of Gleason grade 3 and one biopsy site of Gleason grade 4 only, the global ISUP grade would be 2 (i.e. GS 7[3+4]) or 3 (i.e. GS 7[4+3]), dependent on whether the extent of Gleason grade 3 exceeds that of Gleason grade 4, whereas the worst grade would be ISUP grade 4 (i.e. GS 8[4+4]). Neither global nor worst ISUP grade is clearly superior over the other [348]. The majority of clinical studies have not specified whether global or worst biopsy grade was taken into account. In addition to Gleason score/ISUP grade, the presence/absence of intraductal/invasive cribriform pattern should be reported [110,345,346]. Furthermore, in biopsy Gleason score 7 (ISUP grade 2 and 3) percentage Gleason grade 4 should be monitored at the case and/or biopsy level [110,346]. Lymphovascular invasion (LVI) and EPE must each be reported, if identified, since both carry unfavourable prognostic information [349-351].
Recently, a series of studies have demonstrated that computer-assisted PCa grading artificial intelligence algorithms can perform grading at the level of experienced genito-urinary pathologists. These algorithms have potential in supporting grading of less experienced pathologist, by reducing inter-observer variability, and in quantitative analyses. However, more extensive and prospective validation of these algorithms is needed for implementation in daily clinical practise [110,345,346].
The proportion of systematic (non-targeted) carcinoma-positive cores as well as the extent of tumour involvement per biopsy core correlate with the ISUP grade, tumour volume, surgical margins and pathological stage in RP specimens and predict BCR, post-prostatectomy progression and radiotherapy (RT) failure. These parameters are included in nomograms created to predict pathological stage and SV invasion after RP and RT failure [352,353]. A pathology report should therefore provide both the number of carcinoma-positive cores and the extent of cancer involvement for each core. The length in mm and percentage of carcinoma in the biopsy have equal prognostic impact [354]. An extent of > 50% of adenocarcinoma in a single core is used in some AS protocols as a cut off [355] triggering immediate treatment vs. AS in patients with ISUP grade 1 (see Section 6.1.1.1).
5.2.9.2.2. Recommended item list for reporting prostate cancer biopsies [110, 345, 346]
Type of carcinoma |
Primary and secondary Gleason grade, per biopsy site and global |
International Society of Urological Pathology (ISUP) grade |
Percentage of global Gleason grade 4 in GS 7 biopsies |
Presence/absence of intraductal/invasive cribriform carcinoma |
Number of cancer-positive biopsy cores |
Extent of cancer (in mm or percentage) |
For MRI-targeted biopsies with multiple cores aggreggate (or composite) ISUP grade per lesion |
For carcinoma-negative MRI-targeted biopy, specific benign pathology, e.g. fibrouscular hyperplasia or granulamatous inflammation |
If present, lymphovascular invasion, extra-prostatic extension and ejaculatory duct/seminal vesicle involvement |
5.2.9.3. Tissue-based prognostic biomarker testing
After a comprehensive literature review and several panel discussions an ASCO-EAU-AUA multidisciplinary expert panel made recommendations regarding the use of tissue-based PCa biomarkers. The recommendations were limited to 5 commercially available tests (Oncotype Dx®, Prolaris®, Decipher®, Decipher PORTOS and ProMark®) with extensive validation in large retrospective studies and evidence that their test results might actually impact clinical decision-taking [356]. The selected commercially available tests significantly improved the prognostic accuracy of clinical multivariable models for identifying men who would benefit of AS and those with csPCa requiring curative treatment, as well as for guidance of patient management after RP. Few studies showed that tissue biomarker tests and MRI findings independently improved the detection of csPCa in an AS setting, but it remains unclear which men would benefit of both tests. Decipher® test outcome has been associated with presence of intraductal/invasive cribriform carcinoma, but retains independent value in multivariable analysis [357-359]. Since the long-term impact of the use of these commercially available tests on oncological outcome remains unproven and prospective trials are largely lacking, the Panel concluded that these tests should not be offered routinely but only in subsets of patients where the test result provides clinically actionable information, such as for instance in men with favourable intermediate-risk PCa who might opt for AS or men with unfavourable intermediate-risk PCa scheduled for radiotherapy (RT) to decide on treatment intensification with hormonal therapy (HT).
5.2.9.4. Histopathology of radical prostatectomy specimens
5.2.9.4.1. Processing of radical prostatectomy specimens
Histopathological examination of RP specimens describes the pathological stage, histopathological type, grade and surgical margins of PCa. It is recommended that RP specimens are totally embedded to enable assessment of cancer location, multifocality and heterogeneity. For cost-effectiveness, partial embedding may also be considered, particularly for prostates > 60 g. The most widely accepted method includes complete embedding of the posterior prostate and a single mid-anterior left and right section. Compared with total embedding, partial embedding with this method missed 5% of positive margins and 7% of EPE [360].
The entire RP specimen should be inked upon receipt in the laboratory to demonstrate the surgical margins. Specimens are fixed by immersion in buffered formalin for at least 24 hours, preferably before slicing. After fixation, the apex and the base (bladder neck) are removed and cut into (para)sagittal or radial sections; the shave method is not recommended [108]. The remainder of the specimen is cut in transverse,
3–4 mm sections, perpendicular to the long axis of the urethra. The resultant tissue slices can be embedded and processed as whole-mounts or after quadrant sectioning. Whole-mounts provide better topographic visualisation, faster histopathological examination and better correlation with pre-operative imaging, although they are more time-consuming and require specialist handling. For routine sectioning, the advantages of whole mounts do not outweigh their disadvantages.
5.2.9.4.2. Radical prostatectomy specimen report
The pathology report provides essential information on the prognostic characteristics relevant for clinical decision-making (Table 5.6). As a result of the complex information to be provided for each RP specimen, the use of synoptic(-like) or checklist reporting is recommended. Synoptic reporting results in more transparent and complete pathology reporting [361].
Table 5.6: Mandatory elements provided by the pathology report
Histopathological (sub)type |
Type of carcinoma, e.g., conventional acinar adenocarcinoma, (small cell) neuroendocrine cell carcinoma or ductal carcinoma |
Subtype and unusual variants, e.g., pleomorphic giant cell or mucinous |
Histological grade |
Primary (predominant) Gleason grade Secondary Gleason grade Tertiary Gleason grade (if applicable) Global ISUP grade Approximate percentage of Gleason grade 4 or 5 |
Tumour quantitation (optional) |
Percentage of prostate involved Size/volume of dominant tumour nodule |
Pathological staging (pTNM) |
If extraprostatic extension is present:
If applicable, regional lymph nodes:
|
Surgical margins |
If carcinoma is present at the margin:
|
Other |
Presence of lymphovascular/angio-invasion Location of dominant tumour Presence of intraductal carcinoma/cribriform architecture |
5.2.9.4.3. ISUP grade in prostatectomy specimens
Grading of conventional prostatic adenocarcinoma using the Gleason system is the strongest prognostic factor for clinical behaviour and treatment response [109]. The Gleason score is incorporated in nomograms that predict disease-specific survival (DSS) after prostatectomy [362-364]. The ISUP grade in prostatectomy specimens is determined mostly in a similar way as in biopsies, with a minor exception; i.e. the exclusion of minor (< 5%) high-grade components from the ISUP grade. For instance, in a carcinoma almost entirely composed of Gleason grade 3 the presence of a minor (< 5%) Gleason grade 4 or 5 component is not included in the Gleason score (ISUP grade 1), but its presence is commented upon [110]. In case of multifocality the ISUP grade of the index lesion i.e. the tumour having the highest grade, stage or volume, is given.
5.2.9.4.4. Definition of extra-prostatic extension
Extra-prostatic extension is defined as carcinoma mixed with peri-prostatic adipose tissue, or tissue that extends beyond the prostate gland boundaries (e.g., neurovascular bundle, anterior prostate). Microscopic bladder neck invasion is considered EPE. It is useful to report the location and extent of EPE because the latter is related to recurrence risk [365].
There are no internationally accepted definitions of focal or microscopic, vs. non-focal or extensive EPE. Some describe focal as a few glands [366] or < 1 high-power field in one or at most two sections whereas others measure the depth of extent in millimetres [366].
At the apex of the prostate, tumour mixed with skeletal muscle does not constitute EPE. In the bladder neck, microscopic invasion of smooth muscle fibres is not equated to bladder wall invasion, i.e. not as pT4, because it does not carry independent prognostic significance for PCa recurrence and should be recorded as EPE (pT3a) [367,368]. Stage pT4 is assigned when the tumour invades the bladder muscle wall as determined macroscopically [103].
5.2.9.4.5. PCa volume
Although PCa volume at RP correlates with tumour grade, stage and surgical margin status, the independent prognostic value of PCa volume has not been established [366,369-372]. Improvement in prostatic radio-imaging allows more accurate pre-operative measurement of cancer volume. Since the independent value of pathological tumour volume at RP has not been established, reporting of the diameter/volume of the dominant tumour nodule, or a rough estimate of the percentage of cancer tissue, is optional [373].
5.2.9.4.6. Surgical margin status
Surgical margin status is an independent risk factor for BCR. Margin status is positive if tumour cells are in contact with the ink on the specimen surface. Margin status is negative if tumour cells are close to the inked surface [370] or at the surface of the tissue lacking ink. In tissues that have severe crush artefacts, it may not be possible to determine margin status [374].
Surgical margin is separate from pathological stage, and a positive margin is not evidence of EPE [375]. There is evidence for a relationship between margin extent and recurrence risk [376,377]. However, some indication must be given of the multifocality and extent of margin positivity, such as the linear extent in mm of involvement: focal, < 1 mm vs. extensive, > 1 mm [378], or number of blocks with positive margin involvement. Gleason score at the positive margin was found to correlate independently with outcome, and should be reported [361,376,379].
5.3. Diagnosis - Clinical Staging
5.3.1. T-staging
The cT category listed in Table 4.1 (TNM Classification) only relies on DRE findings. Imaging parameters and biopsy results for local staging are, so far, not part of the risk category stratification [380].
5.3.1.1. US-based techniques and CT
Transrectal US has limited accuracy for PCa local staging [381]. More advanced US-based techniques have not yet been tested in large-scale studies. In case of locally-advanced cancers, abdominopelvic US or CT may show rectal or bladder invasion and dilatation of the upper collecting systems [381].
5.3.1.2. MRI
T2-weighted imaging remains the most useful method for local staging on MRI. Pooled data from a meta-analysis showed a sensitivity and specificity of 0.57 (95% CI: 0.49–0.64) and 0.91 (95% CI: 0.88–0.93), 0.58 (95% CI: 0.47–0.68) and 0.96 (95% CI: 0.95–0.97), and 0.61 (95% CI: 0.54–0.67) and 0.88 (95% CI: 0.85–0.91), for EPE, SVI, and overall stage T3 assessment, respectively [382].
Detection of EPE and SVI seems more accurate at high field strength (3 Tesla) [382], while the added value of functional imaging remains debated [382,383].
In 552 men treated by RP at seven different Dutch centres, MRI showed significantly higher sensitivity (51% vs. 12%; p < 0.001), and lower specificity (82% vs. 97%; p < 0.001) than DRE for non-organ confined disease. All risk groups redefined using MRI findings rather than DRE findings showed better BCR-free survival due to improved discrimination and the Will Roger’s phenomenon [384].
Traditionally, EPE/SVI is assessed visually using qualitative signs (e.g., capsular disruption, visible tumour within peri-prostatic fat). Inter-reader agreement with such subjective reading is moderate, with kappa (k) values ranging from 0.41 to 0.68 [385]. The length of tumour capsule contact (LCC) is also a significant predictor of EPE; it has the advantage of being quantitative, although the ideal cut-off value remains debated [386,387]. Several grading systems combining subjective qualitative signs and/or LCC into a score have shown good sensitivity for EPE (0.68–0.82) with substantial inter-reader agreement (κ = 0.63–0.74), but at the expense of decreased specificity (0.71–0.77); none of these scores has shown definitive superiority over the others [388].
Magnetic resonance imaging findings can improve the prediction of the pathological stage when combined with clinical and biopsy data. As a result, several groups developed multivariate risk calculators for predicting EPE/SVI or positive surgical margins [389]. In external validation cohorts, these risk calculators showed significantly better discrimination than nomograms without MRI-based features [390-392]. However, they remain limited by substantial miscalibration and therefore their results must be interpreted with care.
Given its low sensitivity for focal (microscopic) EPE, MRI is not recommended for local staging in low-risk patients. However, MRI can still be useful for treatment planning.
5.3.2. N-staging
5.3.2.1. Computed tomography and MRI
Abdominal CT and T1-T2-weighted MRI indirectly assess nodal invasion by using LN diameter and morphology. However, the size of non-metastatic LNs varies widely and may overlap the size of LN metastases. Usually, LNs with a short axis > 8 mm in the pelvis and > 10 mm outside the pelvis are considered malignant. Decreasing these thresholds improves sensitivity but decreases specificity. As a result, the ideal size threshold remains unclear [393,394]. Computed tomography and MRI sensitivity is less than 40% [395,396]. Detection of microscopic LN invasion by CT is < 1% in patients with ISUP grade < 4 cancer, PSA < 20 ng/mL, or localised disease [393,397].
Diffusion-weighted MRI (DW-MRI) may detect metastases in normal-sized nodes, but a negative DW-MRI cannot rule out the presence of LN metastases, and DW-MRI provides only modest improvement for LN staging over conventional imaging [398].
5.3.2.2. Risk calculators incorporating MRI findings and clinical data
Because CT and MRI lack sensitivity for direct detection of positive LNs, nomograms combining clinical and biopsy findings have been used to estimate the risk of patients harbouring positive LNs [399-401]. Although these nomograms are associated with good performance, they have been developed using systematic biopsy findings and may therefore not be appropriate for patients diagnosed with combined MRI-targeted biopsy and systematic biopsy.
Two models incorporating MRI-targeted biopsy findings and MRI-derived findings recently underwent external validation [364,402]. One model was tested on an external cohort of 187 patients with a prevalence of LN invasion of 13.9% (vs. 16.9% in the development cohort). The C-index was 0.73 (vs. 0.81 in the development cohort); at calibration analysis, the model tended to overpredict the actual risk [402]. The other model was validated in an external multi-centre cohort of 487 patients with a prevalence of 8% of LN invasion (vs. 12.5% in the development cohort). The AUC was 0.79 (vs. 0.81 in the development cohort). Using a risk cut-off of 7% would have avoided LN dissection in 273 (56% of the cohort), while missing LN invasion in 7 patients (2.6% of the patients below the 7% threshold; 18% of the 38 patients with LN invasion) [403]. Therefore, this nomogram and a 7% threshold should be used after MRI-targeted biopsy to identify candidates for extended LN dissection (eLND).
5.3.2.3. Choline PET/CT
In a meta-analysis of 609 patients, pooled sensitivity and specificity of choline PET/CT for pelvic LN metastases were 62% (95% CI: 51–66%) and 92% (95% CI: 89–94%), respectively [404]. In a prospective trial of 75 patients at intermediate risk of nodal involvement (10–35%), the sensitivity was only 8.2% at region-based analysis and 18.9% at patient-based analysis, which is too low to be of clinical value [405]. The sensitivity of choline PET/CT increases to 50% in patients at high risk and to 71% in patients at very high risk [406].
Due of its low sensitivity, choline PET/CT does not reach clinically acceptable diagnostic accuracy for detection of LN metastases, or to rule out a nodal dissection based on risk factors or nomograms (see Section 6.3.4.1.2).
5.3.2.4. Prostate-specific membrane antigen-based PET/CT
Prostate-specific membrane antigen PET/CT uses several different radiopharmaceuticals; most published studies used 68Ga-labelling for PSMA PET imaging, but some used 18F-labelling. PSMA is also an attractive target because of its specificity for prostate tissue, even if the expression in other non-prostatic malignancies or benign conditions may cause incidental false-positive findings [407-411].
A multi-centre prospective phase III imaging trial, investigating men with intermediate- and high-risk PCa who underwent RP and PLND, showed a sensitivity and specificity of 68Ga-PSMA-11 PET of 0.40 (95% CI: 0.34-0.46), and 0.95 (95% CI: 0.92-0.97), respectively [412]. This is line with previous results from prospective, multi-centre studies addressing the accuracy of 68Ga-PSMA and 18F-DCFPyL PET/CT for LN staging in patients with newly diagnosed PCa [413,414]. Comparable results were also demonstrated in a phase II/III prospective, multi-centre study (OSPREY) with a median specificity of 97.9% (95% CI: 94.5–99.4%) and median sensitivity of 40.3% (28.1–52.5%) for pelvic nodal involvement [415]. This suggests that current PSMA-based PET/CT imaging cannot yet replace diagnostic ePLND (see also Section 6.1.2.3.2).
Prostate-specific antigen may be a predictor of a positive PSMA PET/CT. In the primary staging cohort from a meta-analysis, however, no robust estimates of positivity were found [416].
Comparison between PSMA PET/CT and MRI was performed in a systematic review and meta-analysis including 13 studies (n = 1,597) [417]. 68Ga-PSMA was found to have a higher sensitivity and a comparable specificity for staging pre-operative LN metastases in intermediate- and high-risk PCa [418].
PSMA PET/CT has a good sensitivity and specificity for LN involvement, possibly impacting clinical decision-making. In a review and meta-analysis including 37 articles, a subgroup analysis was performed in patients undergoing PSMA PET/CT for primary staging. On a per-patient-based analysis, the sensitivity and specificity of 68Ga-PSMA PET were 77% and 97%, respectively, after eLND at the time of RP. On a per-lesion-based analysis, sensitivity and specificity were 75% and 99%, respectively [416].
In summary, PSMA PET/CT is more sensitive in N-staging as compared to MRI, abdominal contrast-enhanced CT or choline PET/CT; however, small LN metastases, under the spatial resolution of PET (~5 mm), may still be missed.
5.3.2.5. Risk calculators incorporating MRI and PSMA findings
Recently, an international, multi-centre study incorporated PSMA PET into existing nomograms in order to predict pelvic LN metastatic disease in PCa patients. Performance of 3 nomograms was assessed in 757 patients undergoing RARP and ePLND. Addition of PSMA PET to the nomograms substantially improved the discriminative ability of the models yielding cross-validated AUCs of 0.76 (95% CI: 0.70–0.82), 0.77 (95% CI: 0.72–0.83), and 0.82 (95% CI: 0.76–0.87), respectively [419].
5.3.3. M-staging
5.3.3.1. Bone scan
99mTc-Bone scan is a highly sensitive conventional imaging technique, evaluating the distribution of active bone formation in the skeleton related to malignant and benign disease. A meta-analysis showed combined sensitivity and specificity of 79% (95% CI: 73–83%) and 82% (95% CI: 78–85%) at patient level [420]. Bone scan diagnostic yield is significantly influenced by the PSA level, the clinical stage and the tumour ISUP grade [393,421]. A retrospective study investigated the association between age, PSA and GS in 703 newly diagnosed PCa patients who were referred for bone scintigraphy. The incidence of bone metastases increased substantially with rising PSA and upgrading GS [422]. In two studies, a dominant Gleason pattern of 4 was found to be a significant predictor of positive bone scan [423,424]. Bone scanning should be performed in symptomatic patients, independent of PSA level, ISUP grade or clinical stage [393].
5.3.3.2. Fluoride PET/CT, choline PET/CT and MRI
18F-sodium fluoride (18F-NaF) PET or PET/CT, similarly to bone scintigraphy, only assesses the presence of bone metastases. The tracer was reported to have similar specificity and superior sensitivity to bone scintigraphy for detecting bone metastases in patients with newly diagnosed high-risk PCa [425,426]. Inter-observer agreement for the detection of bone metastases was excellent, demonstrating that 18F-NaF PET/CT is a robust tool for the detection of osteoblastic lesions in patients with PCa [427].
It remains unclear whether choline PET/CT is more sensitive than bone scan but it has higher specificity with fewer indeterminate bone lesions [428-430]. Choline PET/CT has also the advantage of detecting visceral and nodal metastases.
Diffusion-weighted whole-body and axial skeleton MRI are more sensitive than bone scan and targeted conventional radiography in detecting bone metastases in high-risk PCa. Whole-body MRI can also detect visceral and nodal metastases; it was shown to be more sensitive and specific than combined bone scan, targeted radiography and abdominopelvic CT [431]. A meta-analysis found that whole-body MRI is more sensitive than choline PET/CT and bone scan for detecting bone metastases on a per-patient basis, although choline PET/CT had the highest specificity [420].
5.3.3.3. PSMA PET/CT
A systematic review including 12 studies (n = 322) reported high variation in 68Ga-PSMA PET/CT sensitivity for initial staging (range 33–99%; median sensitivity on per-lesion analysis 33–92%, and on per-patient analysis 66–91%), with good specificity (per-lesion 82–100%, and per-patient 67–99%), with most studies demonstrating increased detection rates with respect to conventional imaging modalities (bone scan and CT) [432].
In a prospective multi-centre study in patients with high-risk PCa before curative surgery or RT (proPSMA), 302 patients were randomly assigned to conventional imaging or 68Ga-PSMA-11 PET/CT [433]. The primary outcome focused on the accuracy of first-line imaging for the identification of pelvic LN or distant metastases. Accuracy of 68Ga-PSMA PET/CT was 27% (95% CI: 23–31) higher than that of CT and bone scintigraphy (92% [95% CI: 88–95] vs. 65% [95% CI: 60–69]; p < 0.0001). Conventional imaging had a lower sensitivity (38% [95% CI: 24–52] vs. 85% [95% CI: 74–96]) and specificity (91% [95% CI: 85–97] vs. 98% [95% CI: 95–100]) than PSMA PET/CT. Furthermore, 68Ga-PSMA PET/CT scan prompted management change more frequently as compared to conventional imaging (41 [28%] men [95% CI: 21–36] vs. 23 [15%] men [95% CI: 10–22], p = 0.08), with less equivocal findings (7% [95% CI: 4–13] vs. 23% [95% CI: 17–31]) and lower radiation exposure (8.4 mSv vs. 19.2 mSv; p < 0.001) [433].The comparison of whole body MRI and PSMA PET/CT in detecting bone metastases has led to inconclusive opposite results in two small cohorts [418,434].
5.3.4. Summary of evidence and practical considerations on initial N/M staging
The field of non-invasive N- and M-staging of PCa patients is evolving very rapidly. Evidence shows that choline PET/CT, PSMA PET/CT and whole-body MRI provide a more sensitive detection of LN- and bone metastases than the classical work-up with bone scan and abdominopelvic CT. In view of the evidence offered by the randomised, multi-centre proPSMA trial [433], replacing bone scan and abdominopelvic CT by more sensitive imaging modalities may be a consideration in patients with high-risk PCa undergoing initial staging. However, in absence of prospective studies demonstrating survival benefit, caution must be used when taking therapeutic decisions [435]. The prognosis and ideal management of patients diagnosed as metastatic by these more sensitive tests is unknown. In particular, it is unclear whether patients with metastases detectable only with PET/CT or whole-body MRI should be managed using systemic therapies only, or whether they should be subjected to aggressive local and metastases-directed therapies [436].
Results from RCTs evaluating the management and outcome of patients with (and without) metastases detected by choline PET/CT, PSMA PET/CT and MRI are awaited before a recommendation can be made to treat patients based on the results of these tests [437].
5.3.5. Summary of evidence and guidelines for staging of prostate cancer
Summary of evidence | LE |
PSMA PET/CT is more accurate for staging than CT and bone scan for high-risk disease but to date no outcome data exist to inform subsequent management. | 1b |
Recommendations | Strength rating |
Any risk group staging | |
Use pre-biopsy MRI for local staging information. | Weak |
Treatment should not be changed based on PSMA PET/CT findings, in view of current available data. | Strong |
Low-risk localised disease | |
Do not use additional imaging for staging purposes. | Strong |
Intermediate-risk disease | |
In ISUP grade 3, include at least cross-sectional abdominopelvic imaging and a bone-scan for metastatic screening. | Weak |
High-risk localised disease/locally-advanced disease | |
Perform metastatic screening including at least cross-sectional abdominopelvic imaging and a bone-scan. | Strong |
When using PSMA-PET or whole body MRI to increase sensitivity, be aware of the lack of outcome data of subsequent treatment changes. | Strong |
5.4. Estimating life expectancy and health status
5.4.1. Introduction
Evaluation of life expectancy and health status is important in clinical decision-making for screening, diagnosis, and treatment of PCa. Prostate cancer is common in older men (median age 68) and diagnoses in men > 65 will result in a 70% increase in annual diagnosis by 2030 in Europe and the USA [438,439].
Active treatment mostly benefits patients with intermediate- or high-risk PCa and longest expected survival. In localised disease, over 10 years life expectancy is considered mandatory for any benefit from local treatment and an improvement in CSS may take longer to become apparent. Older age and worse baseline health status have been associated with a smaller benefit in PCSM and life expectancy of surgery vs. AS [440]. Although in a RCT the benefit of surgery with respect to death from PCa was largest in men < 65 years of age (RR: 0.45), RP was associated with a reduced risk of metastases and use of androgen deprivation therapy (ADT) among older men (RR: 0.68 and 0.60, respectively) [441]. External beam RT shows similar cancer control regardless of age, assuming a dose of > 72 Gy when using intensity-modulated or image-guided RT [442].
Older men have a higher incidence of PCa and may be under-treated despite the high overall mortality rates [443,444]. Of all PCa-related deaths 71% occur in men aged > 75 years [445], probably due to the higher incidence of advanced disease and death from PCa despite higher death rates from competing causes [446-448]. In the USA, only 41% of patients aged > 75 years with intermediate- and high-risk disease received curative treatment compared to 88% aged 65–74 [449].
5.4.2. Life expectancy
Life expectancy tables for European men are available online: https://ec.europa.eu/eurostat/web/productsdatasets/-/tps00205. Survival may be variable and therefore estimates of survival must be individualised. Gait speed is a good single predictive method of life expectancy (from a standing start, at usual pace, generally over 6 meters). For men at age 75, 10-year survival ranged from 19% < 0.4 m/s to 87%, for > 1.4 m/s [450].
Figure 5.2: Predicted Median Life Expectancy by Age and Gait Speed for males* [450]
 *Figure reproduced with permission of the publisher, from Studenski S, et al. JAMA 2011 305(1)50.
*Figure reproduced with permission of the publisher, from Studenski S, et al. JAMA 2011 305(1)50.
5.4.3. Health status screening
Heterogeneity increases with advancing age, so it is important to use measures other than just age or performance status (PS) when considering treatment options. The International SIOG PCa Working Group recommends that treatment for adults over 70 years of age should be based on a systematic evaluation of health status using the G8 (Geriatric 8) screening tool (see Table 5.7) [159]. This tool helps to discriminate between those who are fit and those with frailty, a syndrome of reduced ability to respond to stressors. Patients with frailty have a higher risk of mortality and negative side effects of cancer treatment [451]. Healthy patients with a G8 score > 14 or vulnerable patients with reversible impairment after resolution of their geriatric problems should receive the same treatment as younger patients. Frail patients with irreversible impairment should receive adapted treatment. Patients who are too ill should receive only palliative treatment (see Figure 5.3) [159]. Patients with a G8 score < 14 should undergo a comprehensive geriatric assessment (CGA) as this score is associated with 3-year mortality. A CGA is a multi-domain assessment that includes co-morbidity, nutritional status, cognitive and physical function, and social supports to determine if impairments are reversible [452]. A systematic review of the effect of geriatric evaluation for older cancer patients showed improved treatment tolerance and completion [453].
The Clinical Frailty Scale (CFS) is another screening tool for frailty (see Figure 5.4) [454]. Although not frequently used in the cancer setting, it is considered to be a common language for expressing degree of frailty. The scale runs from 1 to 9, with higher scores indicating increasing frailty. Patients with a higher CFS score have a higher 30-day mortality after surgery and are less likely to be discharged home [455].
It is important to use a validated tool to identify frailty, such as the G8 or CFS, as clinical judgement has been shown to be poorly predictive of frailty in older patients with cancer [456].
5.4.3.1. Co-morbidity
Co-morbidity is a major predictor of non-cancer-specific death in localised PCa treated with RP and is more important than age [457,458]. Ten years after not receiving active treatment for PCa, most men with a high co-morbidity score had died from competing causes, irrespective of age or tumour aggressiveness [457]. Measures for co-morbidity include: Cumulative Illness Score Rating-Geriatrics (CISR-G) [459,460] (Table 5.8) and Charlson Co-morbidity Index (CCI) [461].
5.4.3.2. Nutritional status
Malnutrition can be estimated from body weight during the previous 3 months (good nutritional status < 5% weight loss; risk of malnutrition: 5–10% weight loss; severe malnutrition: > 10% weight loss) [462].
5.4.3.3. Cognitive function
Cognitive impairment can be screened for using the mini-COG (https://mini-cog.com/) which consists of three-word recall and a clock-drawing test and can be completed within 5 minutes. A score of < 3/5 indicates the need to refer the patient for full cognitive assessment. Patients with any form of cognitive impairment (e.g., Alzheimer’s or vascular dementia) may need a capacity assessment of their ability to make an informed decision, which is an increasingly important factor in health status assessment [463-465]. Cognitive impairment also predicts risk of delirium, which is important for patients undergoing surgery [466].
5.4.3.4. Physical function
Measures for overall physical functioning include: Karnofsky score and ECOG scores [467]. Measures for dependence in daily activities include: Activities of Daily Living (ADL; basic activities) and Instrumental Activities of Daily Living (IADL; activities requiring higher cognition and judgement) [468-470].
5.4.3.5. Shared decision-making
The patient’s own values and preferences should be taken into account as well as the above factors. A shared decision-making process also involves anticipated changes to QoL, functional ability, and a patient’s hopes, worries and expectations about the future [471]. Particularly in older and frail patients, these aspects should be given equal importance to disease characteristics during the decision-making process [472]. Older patients may also wish to involve family members, and this is particularly important where cognitive impairment exists.
5.4.4. Conclusion
Individual life expectancy, health status, frailty, and co-morbidity, not only age, should be central in clinical decisions on screening, diagnostics, and treatment for PCa. A life expectancy of 10 years is most commonly used as a threshold for benefit of local treatment. Older men may be under-treated. Patients aged 70 years of age or older who have frailty should receive a comprehensive geriatric assessment. Resolution of impairments in vulnerable men allows a similar urological approach as in fit patients.
Table 5.7: G8 screening tool (adapted from [473])
Items | Possible responses (score) | |
A | Has food intake declined over the past 3 months due to loss of appetite, digestive problems, chewing, or swallowing difficulties? | 0 = severe decrease in food intake |
1 = moderate decrease in food intake | ||
2 = no decrease in food intake | ||
B | Weight loss during the last 3 months? | 0 = weight loss > 3 kg |
1 = does not know | ||
2 = weight loss between 1 and 3 kg | ||
3 = no weight loss | ||
C | Mobility? | 0 = bed or chair bound |
1 = able to get out of bed/chair but does not go out | ||
2 = goes out | ||
D | Neuropsychological problems? | 0 = severe dementia or depression |
1 = mild dementia | ||
2 = no psychological problems | ||
E | BMI? (weight in kg)/(height in m2) | 0 = BMI < 19 |
1 = BMI 19 to < 21 | ||
2 = BMI 21 to < 23 | ||
3 = BMI > 23 | ||
F | Takes more than three prescription drugs per day? | 0 = yes |
1 = no | ||
G | In comparison with other people of the same age, how does the patient consider his/her health status? | 0.0 = not as good |
0.5 = does not know | ||
1.0 = as good | ||
2.0 = better | ||
H | Age | 0 = > 85 |
1 = 80-85 | ||
2 = < 80 | ||
Total score | 0-17 |
Figure 5.3: Decision tree for health status screening (men > 70 years)** [159]
 Mini-COGTM = Mini-COGTM cognitive test; ADLs = activities of daily living; CIRS-G = Cumulative IllnessRating Score - Geriatrics; CGA = comprehensive geriatric assessment.
Mini-COGTM = Mini-COGTM cognitive test; ADLs = activities of daily living; CIRS-G = Cumulative IllnessRating Score - Geriatrics; CGA = comprehensive geriatric assessment.
* For Mini-COGTM, a cut-off point of < 3/5 indicates a need to refer the patient for full evaluation of potential dementia.
**Reproduced with permission of Elsevier, from Boyle H. J., et al. Eur J Cancer 2019:116; 116 .159
Figure 5.4: The Clinical Frailty Scale version 2.0 * *Permission to reproduce the CFS was granted by the copyright holder.
*Permission to reproduce the CFS was granted by the copyright holder.
Table 5.8: Cumulative Illness Score Rating-Geriatrics (CISR-G)
1 | Cardiac (heart only) |
2 | Hypertension (rating is based on severity; affected systems are rated separately) |
3 | Vascular (blood, blood vessels and cells, marrow, spleen, lymphatics) |
4 | Respiratory (lungs, bronchi, trachea below the larynx) |
5 | ENT (eye, ear, nose, throat, larynx) |
6 | Upper GI (esophagus, stomach, duodenum. Biliar and parcreatic trees; do not include diabetes) |
7 | Lower GI (intestines, hernias) |
8 | Hepatic (liver only) |
9 | Renal (kidneys only) |
10 | Other GU (ureters, bladder, urethra, prostate, genitals) |
11 | Musculo-Skeletal-Integumentary (muscles, bone, skin) |
12 | Neurological (brain, spinal cord, nerves; do not include dementia) |
13 | Endocrine-Metabolic (includes diabetes, diffuse infections, infections, toxicity) |
14 | Psychiatric/Behavioural (includes dementia, depression, anxiety, agitation, psychosis) |
All body systems are scores on a 0 - 4 scale. - 0: No problem affecting that system. - 1: Current mild problem or past significant problem. - 2: Moderate disability or morbidity and/or requires first line therapy. - 3: Severe problem and/or constant and significant disability and/or hard to control chronic problems. - 4: Extremely severe problem and/or immediate treatment required and/or organ failure and/or severe functional impairment. | |
Total score 0-56 | |
5.4.5. Guidelines for evaluating health status and life expectancy
Recommendations | Strength rating |
Use individual life expectancy, health status, and co-morbidity in PCa management. | Strong |
Use the Geriatric-8, mini-COG and Clinical Frailty Scale tools for health status screening. | Strong |
Perform a full specialist geriatric evaluation in patients with a G8 score < 14. | Strong |
Consider standard treatment in vulnerable patients with reversible impairments (after resolution of geriatric problems) similar to fit patients, if life expectancy is > 10 years. | Weak |
Offer adapted treatment to patients with irreversible impairment. | Weak |
Offer symptom-directed therapy alone to frail patients. | Strong |