1. INTRODUCTION
1.1. Aims and scope
The Prostate Cancer (PCa) Guidelines Panel have prepared this guidelines document to assist medical professionals in the evidence-based management of PCa.
It must be emphasised that clinical guidelines present the best evidence available to the experts but following guideline recommendations will not necessarily result in the best outcome. Guidelines can never replace clinical expertise when making treatment decisions for individual patients, but rather help to focus decisions - also taking personal values and preferences/individual circumstances of patients into account. Guidelines are not mandates and do not purport to be a legal standard of care.
1.2. Panel composition
The PCa Guidelines Panel consists of an international multidisciplinary group of urologists, radiation oncologists, medical oncologists, radiologists, pathologists, a geriatrician and a patient representative.
All imaging sections in the text have been developed jointly with the European Society of Urogenital Radiology (ESUR) and the European Association of Nuclear Medicine (EANM). Representatives of the ESUR and the EANM in the PCa Guidelines Panel are (in alphabetical order): Dr. A. Farolfi, Dr. D. Oprea-Lager, Prof.Dr. O. Rouvière and Dr. I.G. Schoots.
All radiotherapy (RT) sections have been developed jointly with the European Society for Radiotherapy & Oncology (ESTRO). Representatives of ESTRO in the PCa Guidelines Panel are (in alphabetical order): Prof.Dr. G. De Meerleer, Prof.Dr. A.M. Henry, Prof.Dr. M.D. Mason and Prof.Dr. T. Wiegel.
The International Society of Urological Pathology is represented by Prof.Dr. T. van der Kwast and Prof.Dr. A. van Leenders.
Dr. S. O’Hanlon, consultant geriatrician, representing the International Society of Geriatric Oncology (SOIG) contributed to the sections addressing life expectancy, health status and quality of life (QoL) in particular.
Dr. E. Briers, expert Patient Advocate Hasselt-Belgium representing the patient voice as delegated by the European Prostate Cancer Coalition/Europa UOMO.
All experts involved in the production of this document have submitted potential conflict of interest statements which can be viewed on the EAU website Uroweb: https://uroweb.org/guideline/prostate-cancer/.
1.3. Available publications
A quick reference document (Pocket guidelines) is available. This is an abridged version which may require consultation together with the full text version. Several scientific publications are available [1,2] as are a number of translations of all versions of the PCa Guidelines. All documents can be accessed on the EAU website: http://uroweb.org/guideline/prostate-cancer/.
1.4. Publication history and summary of changes
1.4.1. Publication history
The EAU PCa Guidelines were first published in 2001. This 2023 document presents a limited update of the 2022 EAU-EANM-ESTRO-ESUR-ISUP-SIOG PCa Guidelines.
1.4.2. Summary of changes
The literature for the complete document has been assessed and updated based upon a review of all recommendations and creation of appropriate GRADE forms. Evidence summaries and recommendations have been amended throughout the current document and several new sections have been added.
All chapters of the 2022 PCa Guidelines have been updated. New data have been included in the following sections, resulting in new sections, and new and revised recommendations:
Table 4.3: EAU risk groups for biochemical recurrence of localised and locally-advanced prostate cancer
Definition | |||
Low-risk | Intermediate-risk | High-risk | |
PSA < 10 ng/mL and GS < 7 (ISUP grade 1) and cT1-2a* | PSA 10–20 ng/mL or GS 7 (ISUP grade 2/3) or cT2b* | PSA > 20 ng/mL or GS > 7 (ISUP grade 4/5) or cT2c* | any PSA any GS (any ISUP grade)* cT3-4* or cN+** |
Localised | Locally advanced | ||
GS = Gleason score; ISUP = International Society of Urological Pathology; PSA = prostate-specific antigen.
* Based on digital rectal examination.
** Based on CT/bone scan.
5.1.5 Guidelines for screening and individual early detection
Recommendations | Strength rating |
In asymptomatic men with a prostate-specific antigen (PSA) level between | Weak |
In asymptomatic men with a PSA level between 3–10 ng/mL and a normal DRE, use one of the following tools for biopsy indication: risk-calculator, provided it is correctly calibrated to the population prevalence; magnetic resonance imaging of the prostate | Strong |
an additional serum, urine biomarker test | Weak |
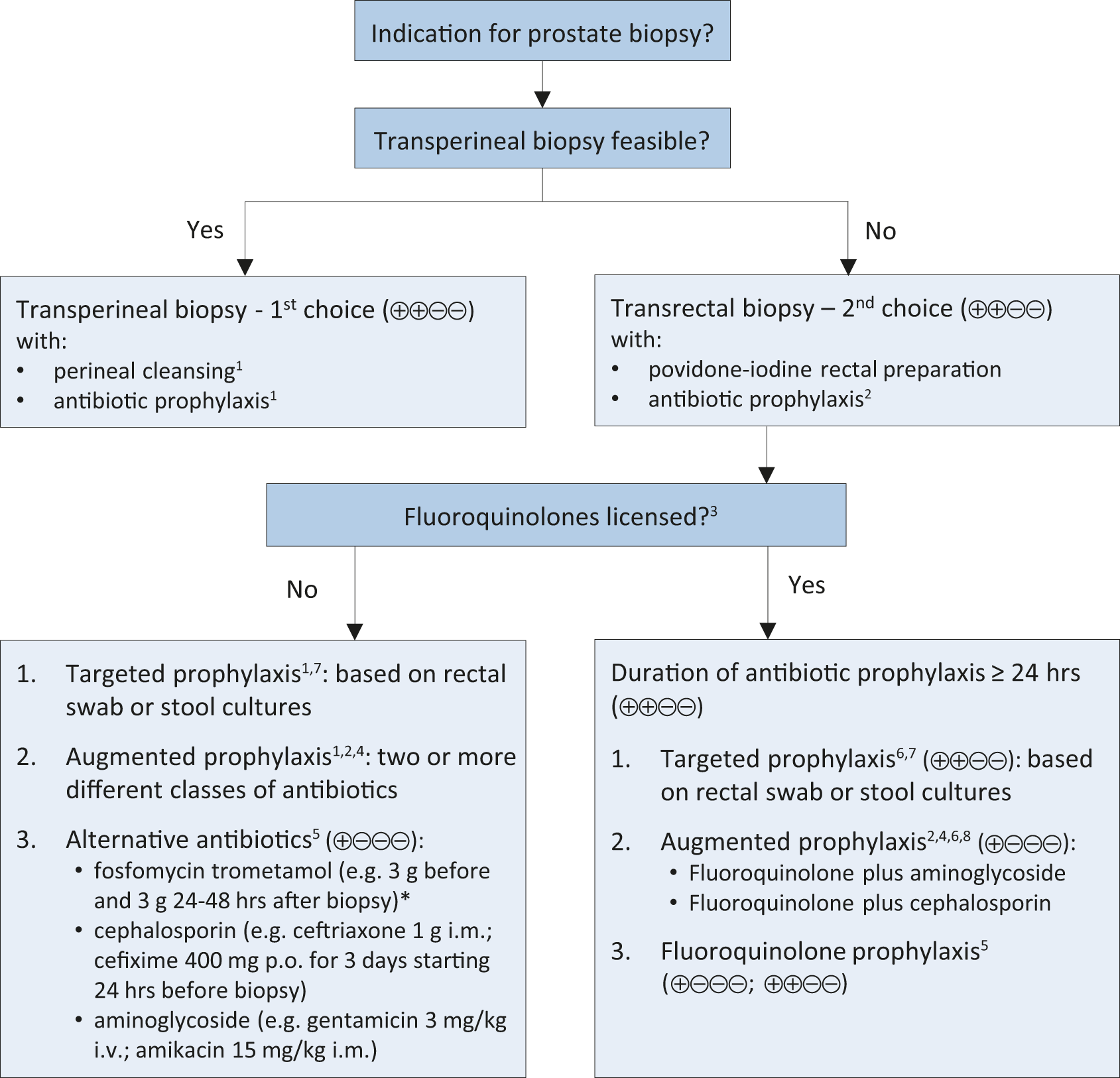
5.2.8.2 Summary of evidence and recommendations for performing prostate biopsy (in line with the EAU Urological Infections Guidelines Panel)
Summary of evidence | LE |
A meta-analysis of eight studies including 1,596 patients showed significantly reduced infectious complications in patients undergoing transperineal biopsy as compared to transrectal biopsy. | 1a |
A meta-analysis of eight non-RCTS reported comparable rates of post-biopsy infections in patients undergoing transperineal biopsy irrespective if antibiotic prophylaxis was given or not. | 1a |
A meta-analysis of eleven RCTs including 2,036 men showed that use of a rectal povidone-iodine preparation before transrectal biopsy, in addition to antimicrobial prophylaxis, resulted in a significantly lower rate of infectious complications. | 1a |
Figure 5.1: Prostate biopsy workflow to reduce infectious complications*
5.3.5 Summary of evidence and guidelines for staging of prostate cancer
Recommendation | Strength rating |
Any risk group staging | |
Treatment should not be changed based on PSMA PET/CT findings, in view of current available data. | Strong |
6.1.6 General guidelines for the treatment of prostate cancer*
Recommendations | Strength rating |
Offer a watchful waiting policy to asymptomatic patients with clinically localised disease and with a life expectancy < 10 years (based on co-morbidities and age). | Strong |
Surgical treatment | |
Radical prostatectomy (RP) can be safely delayed for at least 3 months from diagnosis in any risk category. | Weak |
When a lymph node dissection (LND) is deemed necessary based on a nomogram, perform an extended LND template for optimal staging. | Strong |
Radiotherapeutic treatment | |
Offer moderate hypofractionation (HFX) with IMRT/VMAT plus IGRT to the prostate to patients with localised disease (60 Gy/20 fractions in 4 weeks or 70 Gy/28 fractions in 6 weeks). | Strong |
Offer low-dose rate (LDR) brachytherapy monotherapy to patients with good urinary function and low-risk or NCCN favourable intermediate-risk disease. | Strong |
Offer LDR or high-dose rate (HDR) brachytherapy boost combined with IMRT/VMAT plus IGRT to patients with good urinary function and NCCN unfavourable intermediate-risk or high-risk disease and/or locally-advanced disease. | Weak |
Active therapeutic options outside surgery or radiotherapy | |
Only offer focal therapy with high-intensity focused ultrasound or cryotherapy within a clinical trial or prospective registry. | Strong |
*All recommendations are based on conventional imaging with isotope bone scan and CT/MR abdomen/pelvis.
6.2.1.3 Summary of evidence and guidelines for follow-up during active surveillance
Summary of evidence | LE |
Serial magnetic resonance imaging can improve the detection of aggressive cancers during follow-up. | 3 |
A progression on MRI mandates a repeat biopsy before a change in treatment strategy. | |
A stationary MRI does not make repeat biopsy superfluous. |
Recommendations | Strength rating |
Base follow-up during active surveillance (AS) on a strict protocol including digital rectal examination (at least once yearly), prostate-specific antigen (PSA) (at least once every 6 months) and repeated biopsy every 2 to 3 years. | Strong |
Perform magnetic resonance imaging (MRI) and repeat biopsy if PSA is rising (PSA-doubling time < 3 years). | Strong |
Re-classify patients with low-volume ISUP grade group 2 disease included in AS protocols, if repeat non-MRI-based systematic biopsies performed during monitoring reveal > 3 positive cores or maximum CI > 50%/core of ISUP 2 disease. | Weak |
Base change in treatment on biopsy progression, not on progression on MRI and/or PSA. | Weak |
Patients with a PI-RADS 1-2 findings on MRI and a low PSA density (< 0.15) may be excepted from repeat biopsy. | Weak |
6.2.1.4 Summary of evidence and guidelines for the management of low-risk disease*
Summary of evidence | LE |
Active surveillance or WW is SOC, based on life expectancy. | 2a |
All active treatment options present a risk of over-treatment. | 1a |
Recommendations | Strength rating |
Watchful Waiting | |
Manage patients with a life expectancy < 10 years by watchful waiting. | Strong |
Active surveillance (AS) | |
Manage patients with a life expectancy > 10 years and low-risk disease by AS. | Strong |
*All recommendations are based on conventional imaging with isotope bone scan and CT/MR abdomen/pelvis.
6.2.2.5 Guidelines for the treatment of intermediate-risk disease*
Recommendations | Strength rating |
Watchful Waiting (WW) | |
Offer WW in asymptomatic patients with life expectancy < 10 years. | Strong |
Radical prostatectomy (RP) | |
Offer RP to patients with a life expectancy of > 10 years. | Strong |
Radical prostatectomy can be safely delayed for at least 3 months. | Weak |
Offer nerve-sparing surgery to patients with a low risk of extra-capsular disease on that side. | Strong |
Radiotherapeutic treatment | |
Offer LDR brachytherapy boost combined with IMRT/VMAT plus IGRT to patients with good urinary function and NCCN unfavourable intermediate-risk disease, in combination with short-term ADT (4–6 months). | Weak |
Offer high-dose rate (HDR) brachytherapy boost combined with IMRT/VMAT plus IGRT to patients with good urinary function and NCCN unfavourable intermediate-risk disease, in combination with short-term ADT (4–6 months). | Weak |
Other therapeutic options | |
Only offer whole-gland ablative therapy (such as cryotherapy, high-intensity focused ultrasound, etc.) or focal ablative therapy within clinical trials or registries. | Strong |
*All recommendations are based on conventional imaging with isotope bone scan and CT/MR abdomen/pelvis.
6.2.3.4 Guidelines for radical and palliative treatment of high-risk localised disease*
Recommendations | Strength rating |
Watchful Waiting | |
Offer WW to asymptomatic patients with life expectancy < 10 years. | Strong |
Radical prostatectomy (RP) | |
Radical prostatectomy can be safely delayed for at least 3 months. | Weak |
*All recommendations are based on conventional imaging with isotope bone scan and CT/MR abdomen/pelvis.
6.2.4.5 Guidelines for radical- and palliative treatment of locally-advanced disease*
Recommendations | Strength rating |
Radical prostatectomy (RP) | |
Offer RP to patients with cN0 disease as part of multi-modal therapy. | Weak |
Radiotherapeutic treatment | |
Offer IMRT/VMAT plus IGRT to the prostate in combination with long-term ADT and 2 years of abiraterone to cN0M0 patients with > 2 high-risk factors (cT3-4, Gleason > 8 or PSA > 40 ng/mL). | Strong |
Offer IMRT/VMAT plus IGRT to the prostate plus pelvis in combination with | Strong |
Offer patients with cN1 disease a local treatment (either RP or IMRT/VMAT plus IGRT) plus long-term ADT. | Strong |
*All recommendations are based on conventional imaging with isotope bone scan and CT/MR abdomen/pelvis.
6.2.5.7 Guidelines for adjuvant treatment for pN0 and pN1 disease after radical prostatectomy*
Recommendations | Strength rating |
In pN0 patients with ISUP grade group 4–5 and pT3 ± positive margins, offer adjuvant intensity-modulated radiation therapy (IMRT)/volumetric modulated arc therapy (VMAT) plus image-guided radiation therapy (IGRT). | Strong |
In pN1 patients, after an extended lymph node dissection, discuss three management options, based on nodal involvement characteristics: 1. Offer adjuvant ADT; 2. Offer adjuvant ADT with additional IMRT/VMAT plus IGRT; 3. Offer observation (expectant management) to a patient after eLND and | Weak |
*All recommendations are based on conventional imaging with isotope bone scan and CT/MR abdomen/pelvis.
6.4.8 Guidelines for the first-line treatment of hormone-sensitive metastatic disease*
Recommendations | Strength rating |
At the start of ADT offer luteinising hormone-releasing hormone (LHRH) antagonists or orchiectomy to patients with impending clinical complications like spinal cord compression or bladder outlet obstruction. | Strong |
Offer docetaxel only in combination with ADT plus abiraterone or darolutamide to patients with M1 disease and who are fit for docetaxel. | Strong |
Offer ADT combined with non-curative prostate radiotherapy (using doses up to the equivalent of 72 Gy in 2 Gy fractions) to patients whose first presentation is M1 disease and who have low volume of disease by CHAARTED criteria/M1a disease. | Strong |
*All the following statements are based on metastatic disease defined by bone scintigraphy and CT scan/MRI.
• Section 6.6: Two new flowcharts were introduced
Figure 6.1: Treatment non-metastasized (M0) – asymptomatic disease#

* Rule of thumb: Life expectancy 10 years.
** Recommendation based on clinical staging using digital rectal examination, not imaging.
*** Recommendation based on staging using combination of bone scan and CT.
**** See text, dependent on GG and (biopsy) volume
1EBRT: IMRT/VMAT + IGRT of the prostate
= weak recommendation.
ADT = androgen deprivation therapy; EBRT =external beam radiotherapy; ECE = extracapsular extension; ePLND = extended pelvic lymph node dissection; GG = grade group; HDR = high-dose rate; IDC = intraducal carcinoma; IGRT = image-guided radiotherapy; IMRT = intensity-modulated radiotherapy; LDR = low-dose rate; VMAT = volumetric modulated arc therapy.
Figure 6.2: Treatment of metastasized (M1*) – disease, M+HSPC
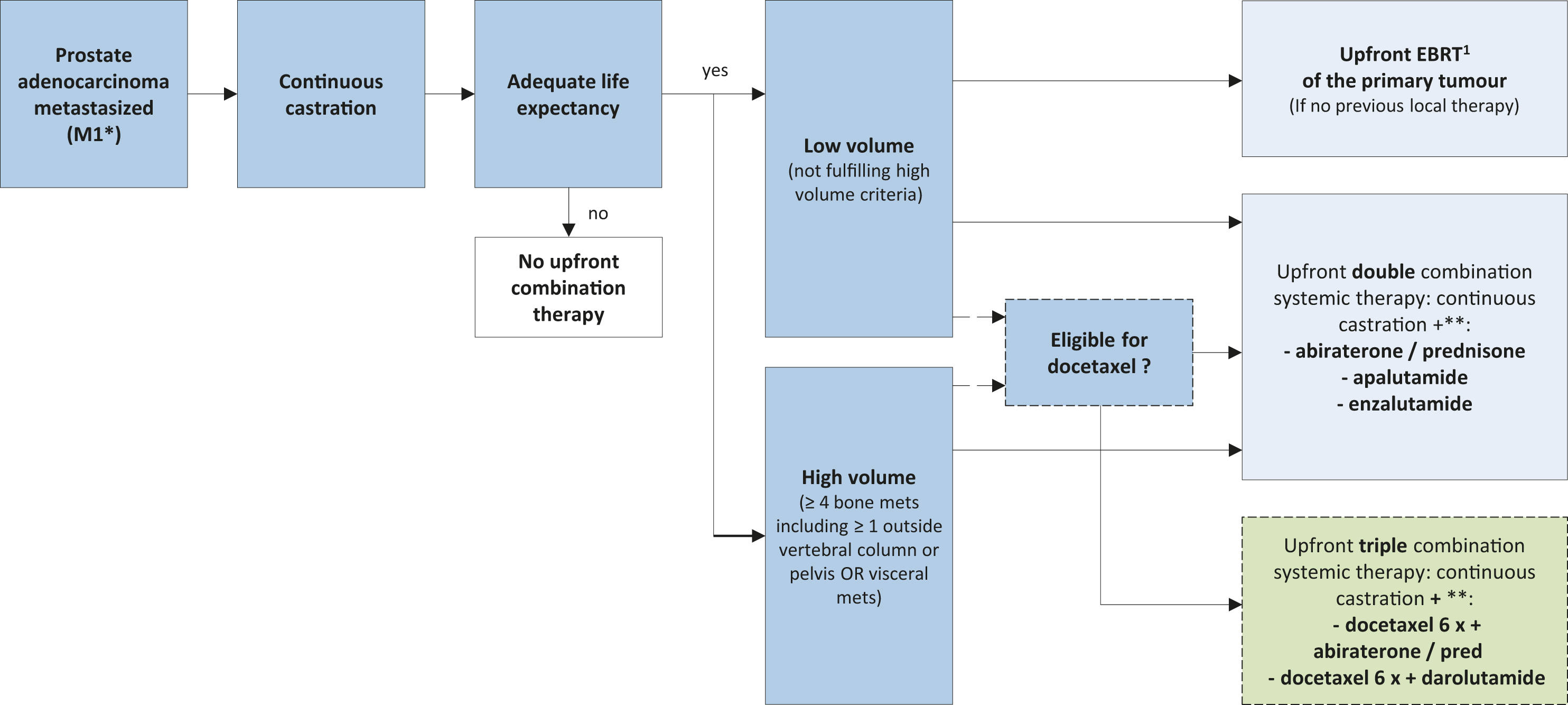
* Based on staging using combination of bone scan and CT.
** Alphabetical order
1EBRT: IMRT/VMAT + IGRT of the prostate (equivalent of up to 72 Gy in 2 Gy fractions).
= weak recommendation.
EBRT = external beam radiotherapy; IGRT = image-guided radiotherapy; IMRT = intensity-modulated radiotherapy.
#Note: Please be aware that the various options in the following flowcharts present a generalised approach
only, and cannot take the management of individual patients into account, nor the availability of resources.
7.1.5 Summary of evidence for follow-up after treatment with curative intent
Summary of evidence | LE |
A detectable PSA, indicating a relapse of the disease, must be differentiated from a clinically meaningful relapse. The PSA threshold that best predicts further metastases after RP is > 0.4 ng/mL and > NADIR + 2 ng/mL after IMRT/VMAT plus IGRT (± ADT]. | 3 |