7. DISEASE MANAGEMENT
7.1. Treatment of localised RCC
7.1.1. Introduction
Sections 7.1.2 and 7.2.4.2 are underpinned by a systematic review which includes all relevant published literature comparing surgical management of localised RCC (T1–2N0M0). Randomised or quasi-RCTs were included. However, due to the very limited number of RCTs, non-randomised studies (NRS), prospective observational studies with controls, retrospective matched-pair studies, and comparative studies from the databases of well-defined registries were also included. Historically, surgery has been the benchmark for the treatment of localised RCC.
7.1.2. Surgical treatment
7.1.2.1. Nephron-sparing surgery versus radical nephrectomy in localised RCC
7.1.2.1.1. T1 RCC
Outcome 1: Cancer-specific survival
Most studies comparing the oncological outcomes of PN and RN are retrospective and include cohorts of varied and, overall, limited size [259,260]. There is only one, prematurely closed, prospective RCT including patients with organ-confined RCCs of limited size (< 5 cm) published, showing comparable non-inferiority of CSS for PN vs. RN (HR: 2.06 [95% CI: 0.62–6.84]) [261].
Outcomes 2 & 3: Overall mortality and renal function
Partial nephrectomy preserved kidney function better after surgery, thereby potentially lowering the risk of development of cardiovascular disorders [259,262-266]. When compared with a radical surgical approach, several retrospective analyses of large databases have suggested a decreased cardiovascular-specific mortality [263,267] as well as improved OS for PN compared to RN. However, in some series this held true only for younger patients and/or patients without significant comorbidity at the time of the surgical intervention [268,269]. An analysis of the U.S. Medicare database [270] could not demonstrate an OS benefit for patients
> 75 years of age when RN or PN were compared with non-surgical management.
Conversely, another series that addressed this question and included Medicare patients, suggested an OS benefit in older patients (75–80 years) when subjected to surgery rather than non-surgical management. Shuch et al., compared patients who underwent PN for RCC with a non-cancer healthy control group via a retrospective database analysis; showing an OS benefit for the cancer cohort [271]. These conflicting results may be an indication that unknown statistical confounders hamper the retrospective analysis of population-based tumour registries. In the only prospectively randomised, but prematurely closed, heavily underpowered, trial, PN seems to be less effective than RN in terms of OS in the intention to treat (ITT) population (HR: 1.50 [95% CI: 1.03–2.16]). However, in the targeted RCC population of the only RCT, the trend in favour of RN was no longer significant [261]. Taken together, the OS advantage suggested for PN vs. RN remains an unresolved issue.
Patients with a normal pre-operative renal function and a decreased GFR due to surgical treatment (either RN or PN), generally present with stable long-term renal function [266]. Adverse OS in patients with a pre-existing GFR reduction does not seem to result from further renal function impairment following surgery, but rather from other medical comorbidities causing pre-surgical chronic kidney disease (CKD) [272]. However, in particular in patients with pre-existing CKD, PN is the treatment of choice to limit the risk of development of ESRD which requires haemodialysis. Huang et al,. found that 26% of patients with newly diagnosed RCC had an GFR < 60 mL/min, even though their baseline serum creatinine levels were in the normal range [105].
Outcomes 4 & 5: Peri-operative outcomes and quality of life
In terms of the intra- and peri-operative morbidity/complications associated with PN vs. RN, the European Organisation for Research and Treatment of Cancer (EORTC) randomised trial showed that PN for small, easily resectable, incidentally discovered RCC, in the presence of a normal contralateral kidney, can be performed safely with slightly higher complication rates than after RN [273].
Only a limited number of studies are available addressing quality of life (QoL) following PN vs. RN, irrespective of the surgical approach used (open vs. minimally invasive). Quality of life was ranked higher following PN as compared to RN, but in general patients’ health status deteriorated following both approaches [273,274].
In view of the above, and since oncological safety (CSS and RFS) of PN, so far, has been found non-differing from RN outcomes, PN is the treatment of choice for T1 RCC since it preserves kidney function better and in the long term PN potentially limits the incidence of cardiovascular disorders and development of ESRD and the need for haemodialysis. Irrespective of the available data, in frail patients, treatment decisions should be individualised, weighing the risks and benefits of PN vs. RN, the increased risk of peri-operative complications, and the risk of developing or worsening of CKD post-operatively.
7.1.2.1.2. T2 RCC
There is very limited evidence on the optimal surgical treatment for patients with larger renal masses (T2). Some retrospective comparative studies of PN vs. RN for T2 RCC have been published [275]. A trend for lower tumour recurrence- and CSM is reported in PN groups. The estimated blood loss is reported to be higher for PN groups, as is the likelihood of post-operative complications [275]. A recent multicentre study compared the survival outcomes in patients with larger (> 7 cm) ccRCC treated with PN vs. RN with long-term follow-up (median 102 months). Compared to the RN group, the PN group had a significantly longer median OS
(p = 0.014) and median CSS (p = 0.04) [276]. Retrospective comparative studies of cT1 and cT2 RCC patients upstaged to pT3a RCC show contradictory results: some reports suggest similar oncologic outcomes between PN and RN [277], whilst another recent report suggests that PN of clinical T1 in pathologically upstaged pT3a of cT1 RCC is associated with a significantly shorter RFS than RN [278]. Overall, the level of the evidence is low. These studies including T2 masses all have a high risk of selection bias due to imbalance between the PN and RN groups regarding patient’s age, comorbidities, tumour size, stage, and tumour position. These imbalances in covariation factors may have a greater impact on patient outcome than the choice of PN or RN. The Panel’s confidence in the results is limited and the true effects may be substantially different.
In view of the above, the risks and benefits of PN should be discussed with patients with T2 tumours. In this setting PN should be considered, if technically feasible, in patients with a solitary kidney, bilateral renal tumours or CKD with sufficient parenchymal volume preserved to allow sufficient post-operative renal function.
7.1.2.1.3. T3 RCC
A recent meta-analysis of nine articles including 1,278 patients with PN and 2,113 patients with RN for pT3a RCC showed no difference in CSS, OS, CSM and RFS, indicating that PN techniques can be used for functional benefits and if technically feasible [279].
7.1.2.2. Associated procedures
7.1.2.2.1. Adrenalectomy
One prospective NRS compared the outcomes of RN with, or without, ipsilateral adrenalectomy [280]. Multivariable analysis showed that upper pole location was not predictive of adrenal involvement, but tumour size was. No difference in OS at five or ten years was seen with, or without, adrenalectomy. Adrenalectomy was justified using criteria based on radiographic- and intra-operative findings. Only 48 of 2,065 patients underwent concurrent ipsilateral adrenalectomy of which 42 of the 48 interventions were for benign lesions [280].
7.1.2.2.2. Lymph node dissection for clinically negative lymph nodes (cN0)
The indication for LN dissection (LND) together with PN or RN is still controversial [281]. The clinical assessment of LN status is based on the detection of an enlargement of LNs either by CT/MRI or intra-operative palpability of enlarged nodes. Less than 20% of suspected metastatic nodes (cN+) are positive for metastatic disease at histopathological examination (pN+) [282]. Both CT and MRI are unsuitable for detecting malignant disease in nodes of normal shape and size [283]. For clinically positive LNs (cN+) see Section 7.2.2.
Smaller retrospective studies have suggested a clinical benefit associated with a more or less extensive LND, preferably in patients at high risk for lymphogenic spread. In a large retrospective study, the outcomes of RN with, or without, LND, in patients with high-risk non-mRCC were compared using a propensity score analysis. In this study LND was not significantly associated with a reduced risk of distant metastases, cancer-specific or all-cause mortality. The extent of the LND was not associated with improved oncologic outcomes [284]. The number of LN metastases (< / > 4) as well as the intra- and extra-capsular extension of intra-nodal metastasis correlated with the patients´ clinical prognosis in some studies [283,285-287]. Better survival outcomes were seen in patients with a low number of positive LNs (< 4) and no extra-nodal extension. Based on a retrospective Surveillance, Epidemiology and End Results (SEER) database analysis of > 9,000 patients no effects of an extended LND (eLND) on the disease-specific survival (DSS) of patients with pathologically-confined negative nodes was demonstrated [288]. However, in patients with pathologically proven lymphogenic spread (pN+), an increase of 10 for the number of nodes dissected resulted in a 10% absolute increase in DSS.
In addition, in a larger cohort of 1,983 patients, Capitanio et al., demonstrated that eLND results in a significant prolongation of CSS in patients with unfavourable prognostic features (e.g., sarcomatoid differentiation, large tumour size) [289]. As to morbidity related to eLND, a recent retrospective propensity score analysis from a large single-centre database showed that eLND is not associated with an increased risk of Clavien grade > 3 complications. Furthermore, LND was not associated with length of hospital stay or estimated blood loss [290].
Only one prospective RCT evaluating the clinical value of LND combined with surgical treatment of primary RCC has been published so far. With an incidence of LN involvement of only 4%, the risk of lymphatic spread appears to be very low. Recognising the latter, only a staging effect was attributed to LND [282]. This trial included a very high percentage of patients with pT2 tumours, which are not at increased risk for LN metastases. Only 25% of patients with pT3 tumours underwent a complete LND and the LN template used by the authors was not clearly stated.
The optimal extent of LND remains controversial. Retrospective studies suggest that an eLND should involve the LNs surrounding the ipsilateral great vessel and the inter-aortocaval region from the crus of the diaphragm to the common iliac artery. Involvement of inter-aortocaval LNs without regional hilar involvement is reported in up to 35–45% of cases [283,291,292]. At least fifteen LNs should be removed
[289,293]. Sentinel LND is an investigational technique [294,295].
7.1.2.2.3. Embolisation
Before routine nephrectomy, tumour embolisation has no benefit [296,297]. In patients unfit for surgery, or with non-resectable disease, embolisation can control symptoms, including visible haematuria or flank pain [298,299]. These indications will be revisited in Sections 7.2 and 7.3 with cross reference to the summary of evidence and recommendations below.
7.1.2.2.4. Summary of evidence and recommendations for the treatment of localised RCC
Summary of evidence | LE |
The oncological outcome in terms of OS following PN equals that of RN in patients with c/p T1 RCC. | 1b |
Retrospective studies suggest that oncological outcomes are similar following PN vs. RN in patients with larger (> 7 cm) RCC. Post-operative complication rates are higher in PN patients. | 3b |
Ipsilateral adrenalectomy during RN or PN has no survival advantage in the absence of clinically evident adrenal involvement. | 3 |
In patients with localised disease without radiographic evidence of LN metastases, a survival advantage of LND in conjunction with RN is not demonstrated in RCTs. | 2b |
Retrospective studies suggest a clinical benefit associated with LND in high-risk patients. | 2b |
In patients unfit for surgery with massive haematuria or flank pain, embolisation can be a beneficial palliative approach. | 3 |
Recommendations | Strength rating |
Offer surgery to achieve cure in localised renal cell cancer. | Strong |
Offer partial nephrectomy (PN) to patients with T1 tumours. | Strong |
Offer PN to patients with T2 tumours and a solitary kidney or chronic kidney disease, if technically feasible. | Weak |
Do not perform ipsilateral adrenalectomy if there is no clinical evidence of invasion of the adrenal gland. | Strong |
Do not offer an extended lymph node dissection to patients with organ-confined disease. | Weak |
Offer embolisation to patients unfit for surgery presenting with massive haematuria or flank pain. | Weak |
7.1.3. Radical and partial nephrectomy techniques
7.1.3.1. Radical nephrectomy techniques
7.1.3.1.1. Open versus laparoscopic or robotic approach
No RCTs have assessed the oncological outcomes of laparoscopic vs. open RN. A cohort study [300] and a number of retrospective database reviews are available, mostly of low methodological quality, showing similar oncological outcomes even for higher stage disease and locally more advanced tumours [301-303]. A retrospective comparative study with data retrieved from a national database studying the OS of open vs.
minimally-invasive RN (laparoscopic RN or RARN) showed an OS benefit in the minimally-invasive RN group, as well as in hospital stay, re-admission rate, and 30-day and 90-day mortality rate [304]. Based on a systematic review, less morbidity was found for laparoscopic vs. open RN [259].
Data from one RCT [275] and two NRS [305,306] showed a significantly shorter hospital stay and lower analgesic requirement for the laparoscopic RN group as compared with the open group. Convalescence time was also significantly shorter [306]. No difference in the number of patients receiving blood transfusions was observed, but peri-operative blood loss was significantly less in the laparoscopic arm in all three studies [302,305,306]. Surgical complication rates were low with very wide confidence intervals. There was no difference in complications, but operation time was significantly shorter in the open nephrectomy arm. Post-operative QoL scores were similar [305].
Some comparative studies focused on the peri-operative outcomes of laparoscopic vs. RN for renal > T2 tumours. Overall, patients who underwent laparoscopic RN were shown to have lower estimated blood loss, less post-operative pain, shorter length of hospital stay, and convalescence, compared to those who underwent open RN [303,306,307]. Intra-operative and post-operative complications were similar in the two groups and no significant differences in CSS, PFS and OS were reported [303,306,307] (LE: 2b). Another multicentre propensity matched analysis compared laparoscopic- and open surgery for pT3a RCC, showing no significant difference in 3-year RFS between groups [308]. The best approach for laparoscopic RN was the retroperitoneal or transperitoneal approach with similar oncological outcomes in two RTCs [309,310] and one quasi-randomised study [283]. Quality of life variables were similar for both approaches. Hand-assisted vs. standard laparoscopic RN was compared in one quasi-randomised study [311] and one database review and estimated 5-year OS, CSS, and RFS rates were comparable [312]. Duration of surgery was significantly shorter in the hand-assisted approach, while length of hospital stay and time to non-strenuous activities were shorter for the standard laparoscopic RN cohort [311,312]. However, the sample size was small.
7.1.3.1.2. Laparoscopic versus robotic approach
Data of a large retrospective cohort study on robot-assisted laparoscopic vs. laparoscopic RN showed robot-assisted laparoscopic RN was not associated with increased risk of any or major complications but had a longer operating time and higher hospital costs compared with laparoscopic RN [313]. A recent systematic review and meta-analysis of seven studies including 1,832 patients showed no difference between the two approaches in peri-operative outcomes, including operative time, blood loss, conversion rates and complications [314]. A systematic review reported on robot-assisted laparoscopic vs. conventional laparoscopic RN, showing no substantial differences in local recurrence rates, nor in all-cause CSM [315].
7.1.3.1.3. Laparoscopic single port versus laparoscopic multi-port approach
Similar results were seen in observational cohort studies comparing ‘portless’ and 3-port laparoscopic RN, with similar peri-operative outcomes [316,317].
7.1.3.2. Partial nephrectomy techniques
7.1.3.2.1. Open versus laparoscopic approach
Studies comparing laparoscopic and open PN found no difference in PFS [318-321] and OS [320,321] in centres with laparoscopic expertise. However, the oncological safety of laparoscopic vs. open PN has, so far, only been addressed in studies with relatively limited follow-up [308]. However, the higher number of patients treated with open surgery in this series might reflect a selection bias by offering laparoscopic surgery in case of a less complex anatomy [308]. The mean estimated blood loss was found to be lower with the laparoscopic approach [318,320,322], while post-operative mortality, deep vein thrombosis, and pulmonary embolism events were similar [318,320]. Operative time is generally longer with the laparoscopic approach [319,321] and warm ischaemia time is shorter with the open approach [318,320,322,323]. The results for GFR decline are debatable, a RCT reported greater 3–12 month kidney function reduction in the open group [324] whilst in a matched-pair comparison, GFR decline was greater in the laparoscopic PN group in the immediate post-operative period [321], but not after 3.6 years follow-up. In another comparative study, the surgical approach was not an independent predictor for post-operative CKD [323]. Retroperitoneal and transperitoneal laparoscopic PN have similar peri-operative outcomes [325]. Simple tumour enucleation also had similar PFS and CSS rates compared to standard PN and RN in a large study [326]. The feasibility of laparo-endoscopic single-site PN has been shown in selected patients but larger studies are needed to confirm its safety and clinical role [327].
7.1.3.2.2. Open versus robotic approach
One study prospectively compared the peri-operative outcomes of a series of robot-assisted and open PN performed by the same experienced surgeon. Robot-assisted PN was superior to open PN in terms of lower estimated blood loss and shorter hospital stay. Warm ischaemia time, operative time, immediate- early- and short-term complications, variation in creatinine levels and pathologic margins were similar between groups [328].
A multicentre French prospective database compared the outcomes of 1,800 patients who underwent open PN and robot-assisted PN. Although the follow-up was shorter, there was a decreased morbidity in the robot-assisted PN group with less overall complications, less major complications, less transfusions and a much shorter hospital stay [329].
OPERA, a prospective RCT comparing open (OPN) vs. robotic partial nephrectomy (RAPN) in intermediate/high complexity renal tumours (RENAL Score > = 7) prematurely closed due to poor accrual. Considering these limitations, the clinical impact of robotic PN is still controversial.
7.1.3.2.3. Open versus hand-assisted approach
Hand-assisted laparoscopic PN (HALPN) is rarely performed. A recent comparative study of open vs. HALPN showed no difference in OS or RFS at intermediate-term follow-up. The authors observed a lower rate of intra-operative and all-grade post-operative 30-day complications in HALPN vs. open PN patients, but there was no significant difference in high Clavien grade complications. Three months after the operation, GFR was lower in the HALPN than in the open PN group [330].
7.1.3.2.4. Open versus laparoscopic versus robotic approaches
In a retrospective propensity-score-matched study, comparing open-, laparoscopic- and robot-assisted PN, after five years of median follow-up, similar rates of local recurrence, distant metastasis and cancer-related death rates were found [331].
7.1.3.2.5. Laparoscopic versus robotic approach
Another study included the 50 last patients having undergone laparoscopic and robotic PN for T1–T2 renal tumours by two different surgeons with an experience of over 200 procedures each in laparoscopic and robotic PN and RAPN, respectively, at the beginning of the study. Peri-operative and short-term oncological and functional outcomes appeared broadly comparable between RAPN and LPN when performed by highly experienced surgeons [332].
A meta-analysis, including a series of NSS with variable methodological quality compared the peri-operative outcomes of robot-assisted- and laparoscopic PN. The robotic group had a significantly lower rate of conversion to open surgery and to radical surgery, shorter warm ischaemia time, smaller change in estimated GFR after surgery and shorter length of hospital stay. No significant differences were observed between the two groups regarding complications, change of serum creatinine after surgery, operative time, estimated blood loss and positive surgical margins (PSMs) [333].
A recent multi-institutional prospective study of 105 patients with hilar tumours demonstrated a reduced warm ischaemia time (20.2 min vs. 27.7 min) and a comparable rate of 1.9% when compared with a historical laparoscopic control group which was defined by literature research and meta-analysis for warm ischaemia time and PSM, respectively [334].
7.1.3.2.6. Laparoscopic transperitoneal versus retroperitoneal approach
Data from the Italian RECORD 2 project, a multi-institutional prospective observational project, compared the transperitoneal vs. the retroperitoneal approach for laparoscopic PN. After propensity score matching (each group n = 413) no differences in post-operative complications (surgical and medical), PSMs, early and late eGFR levels were observed. Intra-operative and surgical complications were slightly higher and operative times lower in the transperitoneal vs. the retroperitoneal approach [335]. In terms of peri-operative complications, retroperitoneal and transperitoneal PN have similar outcomes [335].
A recent systematic review assessed the outcomes of retroperitoneal vs. transperitoneal RAPN. Seventeen studies, published between 2013 and 2021, were retrieved; none of which a RCT. Among the 6,266 patients included 2,261 (36.1%) and 4,005 (63.9%) underwent retroperitoneal vs. transperitoneal RAPN, respectively. Both retroperitoneal and transperitoneal RAPN offered similar surgical outcomes, while retroperitoneal RAPN was associated with shorter surgical time and length of hospital stay [336].
7.1.3.2.7. Tumour enucleation, standard partial nephrectomy and single-port approach
Simple tumour enucleation also had similar PFS and CSS rates compared to standard PN and RN in a large study [326]. The feasibility of laparo-endoscopic single-site PN has been shown in selected patients but larger studies are needed to confirm its safety and clinical role [327].
The only prospective multi-centre study available to date assessing the impact of resection technique (enucleation vs. enucleoresection vs. resection) during PN using a standardised reporting score to classify the resection technique after surgery found that the resection technique significantly impacts surgical complications, early functional outcomes and positive surgical margins after PN of localised renal masses [337].
7.1.3.2.8. Surgical volume
In a recent analysis of 8,753 patients who underwent PN, an inverse non-linear relationship of hospital volume with morbidity of PN was observed, with a plateauing seen at 35 to 40 cases per year overall, and 18 to 20 cases for the robotic approach [338]. A retrospective study of a U.S. National Cancer Database looked at the prognostic impact of hospital volume and the outcomes of robot-assisted PN including 18,724 cases. This study shows that undergoing RAPN at higher-volume hospitals may have better peri-operative outcomes (conversion to open and length of hospital stay) and lower PSM rates [339]. A French study, including 1,222 RAPN patients, has shown that hospital volume is the main predictive factor of Trifecta achievement (no complications, warm ischaemia time < 25 min, and negative surgical margins) after adjustment for other variables, including surgeon volume [340]. The prospective Registry of Conservative and Radical Surgery for cortical renal tumour Disease (RECORd-2) study including 2,076 patients showed that the hospital volume (> 60 PN/year) is an independent predictor for PSMs [341].
7.1.3.2.9. Pre-operative embolisation prior to partial nephrectomy
A systematic review and meta-analysis of 270 patients demonstrated significantly reduced blood loss in patients with selective renal artery embolisation (n = 222; 154 ± 22.6 mL vs. n = 48; 353.4 ± 69.6 mL) prior to PN [342].
7.1.3.3. Positive surgical margins on histopathological specimens
A PSM is encountered in about 2–8% of PNs [333]. Studies comparing surgical margins with different surgical approaches (open, laparoscopic, robotic) are inconclusive [343,344]. Most trials showed that intra-operative frozen section analysis had no influence on the risk of definite PSMs [345]. A PSM status occurs more frequently in cases in which surgery is imperative (solitary kidneys and bilateral tumours) and in patients with adverse pathological features (pT2a, pT3a, grade III–IV) [346-349].
The majority of retrospective analyses reported so far indicated that PSMs do not translate into a higher risk of metastases or a decreased CSS [347,348]. On the other hand, another retrospective study of a large single-institutional series showed that PSMs are an independent predictor of PFS due to a higher incidence of distant and local relapses [350]. Another retrospective study of 42,114 PN patients with 2,823 PSM patients (6.7%) showed an increased presence of PSM in upstaged pT3a tumours (14.1%), increased all-cause mortality in PSM patients and a decreased 5-year OS rate in pT3a tumours (PSM: 69% vs. NSM: 90.9 %) [351].
However, only a proportion of patients with an uncertain margin status actually harbour residual malignancy [352]. Local tumour bed recurrences were found in 16% in patients with PSMs compared with 3% in those with negative margins [346], Therefore, RN or re-resection of margins can result in over-treatment in many cases. Patients with PSMs should be informed that they will need a more intense surveillance (imaging) follow-up and that they are at increased risk of secondary local therapies [347,353]. On the other hand, protection from recurrence is not ensured by negative surgical margins [354].
7.1.3.4. Summary of evidence and recommendations for radical and partial nephrectomy techniques
Summary of evidence | LE |
Laparoscopic RN has lower morbidity than open nephrectomy. | 1b |
Short-term oncological outcomes for T1–T2a tumours are equivalent for laparoscopic- and open RN. | 2a |
Partial nephrectomy can be performed, either by open-, pure laparoscopic- or robot-assisted approach, based on surgeon’s expertise and skills. | 2b |
Robot-assisted and laparoscopic PN are associated with shorter length of hospital stay and lower blood loss compared to open PN. | 2b |
Partial nephrectomy is associated with a higher percentage of PSMs compared to RN. | 3 |
Transperitoneal and retroperitoneal laparoscopic PN do not differ in in post-operative surgical and medical complications, PSMs, and kidney function. | 2a |
Hospital volume for PN might impact on surgical complications, warm ischaemia time and surgical margins. | 3 |
Radical nephrectomy after PSMs can result in over-treatment in many cases. | 3 |
Recommendations | Strength rating |
Offer laparoscopic radical nephrectomy (RN) to patients with T2 tumours and localised masses not treatable by partial nephrectomy (PN). | Strong |
Do not perform minimally-invasive RN in patients with T1 tumours for whom a PN is feasible by any approach, including open. | Strong |
Do not perform minimally-invasive surgery if this approach may compromise oncological-functional- and peri-operative outcomes. | Strong |
Intensify follow-up in patients with a positive surgical margin, especially in upstaged pT3a patients. | Weak |
7.1.4. Therapeutic approaches as alternatives to surgery
7.1.4.1. Active surveillance and watchful waiting
Elderly and comorbid patients with incidental SRMs have a low RCC-specific mortality and significant competing-cause mortality [355,356]. Active surveillance is defined as the initial monitoring of tumour size by serial abdominal imaging (US, CT, or MRI) with delayed intervention reserved for tumours showing clinical progression during follow-up [357]. The concept of AS differs from the concept of ‘Watchful Waiting’; Watchful Waiting is reserved for patients whose comorbidities contra-indicate any subsequent active treatment and who do not require follow-up imaging, unless clinically indicated.
Population-based studies compared the oncological outcomes of surgery (RN or PN) and non-surgical management for tumours < 4 cm. The analyses showed a significantly lower CSM in patients treated with surgery [270,358,359]. However, the patients assigned to the surveillance arm were older and likely to be frailer and less suitable for surgery. Other-cause mortality rates in the non-surgical group significantly exceeded that of the surgical group [358]. Analyses of older patients (> 75 years) failed to show the same benefit in CSM for surgical treatment [360-362].
Growth rate and metastasis
In the largest reported series of AS the growth of renal tumours was low and progression to metastatic disease was reported in only a limited number of patients [363,364]. A systematic review of eighteen AS cohorts comprising 2,066 patient (cT1–2 N0M0) with a pooled mean follow-up of 53 months, showed that 2.1% (95% CI: 1.0–3.6) of patients developed metastatic disease during follow-up [365]. For patients with SRMs (nine studies, n = 987), the pooled metastasis rate was 1.8% (95% CI: 0.5–3.7).
In 136 biopsy-proven SRMs managed by AS, median follow-up of patients who remained on AS was 5.8 years (interquartile range 3.4-7.5 years). Clear-cell RCC grew faster than papillary type 1 SRMs (0.25 and 0.02 cm/year on average, respectively, p = 0.0003). Overall, 60 (44.1 %) of the malignant SRMs progressed; 49 (82%) by rapid growth (volume doubling), seven (12%) increasing to > 4 cm, and four (6.7%) by both criteria. Six patients developed metastases, and all were of ccRCC histology [366].
Overall- and cancer-specific survival
A single-institutional comparative study evaluating patients aged > 75 years showed decreased OS for those who underwent surveillance and nephrectomy relative to NSS for clinically T1 renal tumours. However, at multivariate analysis, management type was not associated with OS after adjusting for age, comorbidities, and other variables [355]. No statistically significant differences in OS and CSS were observed in another study of RN vs. PN vs. AS for T1a renal masses with a follow-up of 34 months [367].
The prospective non-randomised multi-institutional Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) study enrolled 497 patients with solid renal masses < 4 cm who selected either AS or primary active intervention. Patients who selected AS were older, had worse ECOG scores, more comorbidities, smaller tumours, and more often had multiple and bilateral lesions. In patients who elected AS in this study the overall median SRM growth rate was 0.09 cm/year with a median follow-up of 1.83 years. The growth rate and variability decreased with longer follow-up. No patients developed metastatic disease or died of RCC [368,369].
Overall survival for primary intervention and AS was 98% and 96% at two years, and 92% and 75% at five years, respectively (p = 0.06). At five years, CSS was 99% and 100%, respectively (p = 0.3). Active surveillance was not predictive of OS or CSS in regression modelling with relatively short follow-up [368]. In the previously mentioned large systematic review of eighteen AS cohorts 1.0% (95% CI: 0.3–2.1) died from RCC and 22.6% (95% CI: 15.8–30.2) died from any cause. For patients with SRMs RCC-specific mortality was 0.6% (95% CI: 0–2.1), and all-cause mortality was 28.5% (95% CI: 17.4–41.4) [365].
Overall, both short- and intermediate-term oncological outcomes indicate that in selected patients with advanced age and/or comorbidities, AS is appropriate for initially monitoring of SRMs, followed, if required, by treatment for progression [357,363,364,370-373].
Quality of life
A multicentre study assessed QoL of patients undergoing immediate intervention vs. AS. Patients undergoing immediate intervention had higher QoL scores at baseline, specifically for physical health. The perceived benefit in physical health persisted for at least one year following intervention. Mental health, which includes domains of depression and anxiety, was not adversely affected while on AS [374].
7.1.4.2. Role of renal tumour biopsy before active surveillance
Histological characterisation of SRMs by renal tumour biopsy is useful to select tumours at lower risk of progression based on grade and histotype, which can be safely managed with AS. Pathology can also help to tailor surveillance imaging schedules. In the largest cohort of biopsy-proven, small, sporadic RCCs followed with AS, a significant difference in growth and progression among different RCC subtypes was observed. Clear-cell RCC SRMs grew faster than papillary type 1 SRMs (0.25 and 0.02 cm/year on average, respectively, p = 0.0003) [366].
7.1.4.3. Tumour ablation
7.1.4.3.1. Role of renal mass biopsy
A RMB is required prior to tumour ablation (TA) (see Sections 5.3 - Renal tumour biopsy and 5.4 - Summary of evidence and recommendations for the diagnostic assessment of RCC). Historically, up to 45% of patients underwent TA of a benign or non-diagnostic mass [375,376]. An analysis of the European multi-national prospective EuRECA registry (871 patients undergoing cryoablation) showed that the use of pre-cryoablation biopsy has significantly increased from 42% (65/156) in 2015 to 72% (88/122) in 2019 (p < 0.001), making treatment for a benign or an unknown histology significantly less likely (OR: 0.64, p < 0.001 and OR 0.31, p = 0.044, respectively) [377]. A RMB in a separate session reduces over-treatment significantly, with 80% of patients with benign lesions opting not to proceed with TA [376]. Additionally, there is some evidence that the oncological outcome following TA differs according to RCC subtype which should therefore be factored into the decision-making process. In a series of 229 patients with cT1a tumours (mean size 2.5 cm) treated with RFA, the 5-year DFS rate was 90% for ccRCC and 100% for pRCC (80 months: 100% vs. 87%, p = 0.04) [378]. In another series, the total TA effectiveness rate was 90.9% for ccRCC and 100% for pRCC [379]. A study comparing RFA with surgery suggested worse outcomes of RFA vs. PN in cT1b ccRCC, while no difference was seen in those with non-ccRCC [380]. Furthermore, patients with high-grade RCC or metastasis may choose different treatments over TA. Finally, patients without biopsy or a non-diagnostic biopsy are often assumed to have RCC and will undergo potentially unnecessary radiological follow-up or further treatment.
7.1.4.3.2. Cryoablation
Cryoablation is performed using either a percutaneous- or a laparoscopic-assisted approach, with technical success rates of > 95% [381]. In comparative studies, there was no significant difference in the overall complication rates between laparoscopic- and percutaneous cryoablation [382-384]. One comparative study reported similar OS, CSS, and RFS in 145 laparoscopic patients with a longer follow-up vs. 118 patients treated percutaneously with a shorter follow-up [383]. A shorter average length of hospital stay was found with the percutaneous technique [383-385]. A systematic review including 82 articles reported complication rates ranging between 8 and 20% with most complications being minor [386]. Although a precise definition of tumour recurrence is lacking, the authors reported a lower RFS as compared to that of PN.
Oncological outcomes after cryoablation have generally been favourable for cT1a tumours. In a recently published series of 308 patients with cT1a and cT1b tumours undergoing percutaneous cryoablation, local recurrence was seen in 7.7% of cT1a tumours vs. 34.5% of cT1b tumours. On multivariable regression, the risk of disease progression increased by 32% with each 1 cm increase in tumour size (HR: 1.32, p < 0.001). Mean decline in eGFR was 11.7 mL/min/1.73 m2 [387]. In another large series of 220 patients with biopsy-proven cT1 RCC, 5-year local RFS was 93.9%, while metastasis-free survival approached 94.4% [381]. A series of 134 patients with T1 RCC (median tumour size 2.8 cm) submitted to percutaneous cryoablation yielded a 10-year DSF of 94% [388].
For cT1b tumours, local tumour control rates drop significantly. One study showed local tumour control in only 60.3% at three years [389]. In another series, the PFS rate was 66.7% at twelve months [390]. Furthermore, recent analyses demonstrated 5-year CSM rates of 7.6–9% [391,392]. On multivariable analysis, cryoablation of cT1b tumours was associated with a 2.5-fold increased risk of death from RCC compared with PN [391].
Recurrence after initial cryoablation is often managed with re-cryoablation, but only 45% of patients remain disease-free at two years [393].
7.1.4.3.3. Radiofrequency ablation
Radiofrequency ablation is performed laparoscopically or percutaneously. Several studies compared patients with cT1a tumours treated by laparoscopic or percutaneous RFA [394-397]. Complications occurred in up to 29% of patients but were mostly minor. Complication rates, recurrence rates and CSS were similar in patients treated laparoscopically and percutaneously.
The initial technical success rate on early (i.e., one month) imaging after one session of RFA is 94% for cT1a and 81% for cT1b tumours [398]. This is generally managed by re-RFA, approaching overall total technical success rates > 95% with one or more sessions [399].
Long-term outcomes with over five years of follow-up following RFA have been reported. In recent studies, the 5-year OS rate was 73–79% [398,399], due to patient selection. Oncological outcomes for cT1a tumours have been favourable. In a recent study, the 10-year DFS rate was 82%, but there was a significant drop to 68% for tumours > 3 cm [399]. In series focusing on clinical T1b tumours (4.1–7.0 cm), the 5-year DFS rate was 74.5% to 81% [398,400]. Oncological outcomes appear to be worse than after surgery, but comparative data are severely biased (see Section 7.1.4.3.4). In general, most disease recurrences occur locally and recurrences beyond five years are rare [399,400].
7.1.4.3.4. Tumour ablation versus surgery
The Guideline Panel performed a protocol-driven systematic review of comparative studies (including > 50 patients) of TA with PN for T1N0M0 renal masses [401]. Twenty-six non-randomised comparative studies published between 2000 and 2019 were included, recruiting a total of 16,780 patients. Four studies compared laparoscopic TA vs. laparoscopic/robotic PN; sixteen studies compared laparoscopic or percutaneous TA vs. open-, laparoscopic- or robotic PN; two studies compared different techniques of TA and four studies compared TA vs. PN vs. RN. In this systematic review, TA as treatment for T1 renal masses was found to be safe in terms of complications and adverse events (AEs), but its long-term oncological effectiveness compared with PN remained unclear. The primary reason for the persisting uncertainty was related to the nature of the available data; most studies were retrospective observational studies with poorly matched controls, or single-arm case series with short follow-up. Many studies were poorly described and lacked a clear comparator. There was also considerable methodological heterogeneity. Another major limitation was the absence of clearly defined primary outcome measures. Even when a clear endpoint such as OS was reported, data were difficult to interpret because of the varying length and type of follow-up amongst studies. The Panel also appraised the published systematic reviews based on the AMSTAR 2 tool which showed “Critically Low” or “Low” ratings [401].
Tumour ablation has been demonstrated to be associated with good long-term survival in several single-arm non-comparative studies [402,403]. Due to the lack of controls, this apparent benefit is subject to significant uncertainties. Whether such benefit is due to the favourable natural history of such tumours or due to the therapeutic efficacy of TA, as compared to PN, remains unknown. In addition, there are data from comparative studies suggesting TA may be associated with worse oncological outcomes in terms of local recurrence and metastatic progression and CSM [268,391,392,404,405]. However, there appears to be no clinically significant difference in 5-year CSM between TA and AS [359].
The Panel concluded that the current data are inadequate to reach conclusions regarding the clinical effectiveness of TA as compared with PN. Given these uncertainties in the presence of only low-quality evidence, TA can only be recommended to frail and/or comorbid patients with SRMs.
7.1.4.3.5. Stereotactic ablative radiotherapy
Stereotactic ablative radiotherapy (SABR) has been emerging as a treatment option for medically inoperable patients with localised cT1a and cT1b tumours. Patients usually receive 26 Gy in a single fraction, three fractions of 14 Gy or five fractions of 6 Gy [406,407]. In a systematic review of non-comparative single-arm studies with a median follow-up range of 5.8–79.2 months, the local control rate was 97.2% and the mean change in eGFR was 7.7 mL/min/1.73 m2. Grade 3 or 4 toxicities occurred in 1.5% of patients. However, viable tumour cells are often seen in post-SABR biopsies, although their clinical significance remains unclear [407]. Even though early results of SABR are encouraging, more evidence from RCTs is needed [408].
7.1.4.3.6. Microwave ablation
The best evidence base for these techniques exists for percutaneous microwave ablation. In a study of 185 patients with a median follow-up of 40 months, the 5-year local progression rate was 3.2%, while 4.3% developed distant metastases [409]. Results appear to be favourable for cT1b tumours as well [410]. Overall, current data on cryoablation, RFA and microwave ablation of cT1a renal tumours indicate short-term equivalence with regards to complications, oncological- and renal functional outcomes [411].
7.1.4.3.7. Other ablative techniques
Some studies have shown the feasibility of other ablative techniques, such as high-intensity focused US ablation and non-thermal irreversible electroporation. However, these techniques are still considered experimental.
7.1.4.3.8. Summary of evidence and recommendation for therapeutic approaches as alternative to surgery
Summary of evidence | LE |
Most population-based analyses show a significantly lower CSM for patients treated with surgery compared to non-surgical management. | 3 |
In AS cohorts, the growth of SRMs is low in most cases and progression to metastatic disease is rare (1–2%). | 3 |
Low-quality studies suggest higher disease recurrence rates after RFA of tumours > 3 cm and after cryoablation of tumours > 4 cm. | 3 |
Low-quality studies suggest a higher local recurrence rate for TA therapies compared to PN, but quality of data does not allow definitive conclusions. | 3 |
Recommendations | Strength rating |
Offer active surveillance (AS) or thermal ablation (TA) to frail and/or comorbid patients with small renal masses. | Weak |
Perform a percutaneous renal mass biopsy prior to, and not concomitantly with, TA. | Strong |
When TA or AS are offered, discuss with patients about the harms/benefits with regards to oncological outcomes and complications. | Strong |
Do not routinely offer TA for tumours > 3 cm and cryoablation for tumours > 4 cm. | Weak |
7.2. Treatment of locally advanced RCC
7.2.1. Introduction
In addition to the summary of evidence and recommendations outlined in Section 7.1 for localised RCC, certain therapeutic strategies arise in specific situations for locally-advanced disease.
7.2.2. Role of lymph node invasion in locally-advanced RCC
In locally-advanced RCC, the role of LND is still controversial. The only available RCT demonstrated no survival benefit for patients undergoing LND but this trial mainly included organ-confined disease cases [282]. In the setting of locally-advanced disease, several retrospective papers and systematic reviews addressed the topic with contradictory results. Bhindi et al., could not confirm any survival benefit in patients at high risk of progression treated with LND [412]. More recently, Luo et al., reported a systematic review and meta-analyses showing a survival benefit in patients with locally-advanced disease treated with LND [413]. More specifically, thirteen studies on patients with LND and non-LND were identified and included in the analysis. In the subgroup of locally-advanced RCC (cT3–T4NxM0), LND showed a significantly better OS rate in patients who had undergone LND compared to those without LND (HR: 0.73, 95% CI: 0.60–0.90, p = 0.003).
7.2.2.1. Management of clinically negative lymph nodes (cN-) in locally-advanced RCC
In case of cN-, the probability of finding pathologically-confirmed LN metastases ranges between 0 and 25%, depending mainly on primary tumour size and the presence of distant metastases [414]. In case of clinically-negative LNs (cN-) at imaging, removal of LNs is justified only if visible or palpable during surgery [415], at least for staging, prognosis, adjuvant therapy and follow-up implications, although a benefit in terms of cancer control has not yet been demonstrated [284,412]. Whether to extend the LND also to retroperitoneal areas without cN+ remains controversial [283].
7.2.2.2. Management of clinically positive lymph nodes (cN+) in locally-advanced RCC
In case of cN+, the probability to identify pathologically-confirmed LN metastases ranges between 10.3% (cT1 tumours) up to 54.5% in case of locally-advanced disease. In cN+, removal of visible and palpable nodes during LND is always justified [415], at least for staging, prognosis, adjuvant therapy and follow-up implications, although a benefit in terms of cancer control has not yet been demonstrated [284,412].
7.2.3. Management of RCC with venous tumour thrombus
Tumour thrombus formation in RCC patients is a significant adverse prognostic factor. Traditionally, patients with venous tumour thrombus undergo surgery to remove the kidney and tumour thrombus. Aggressive surgical resection is widely accepted as the default management option for patients with venous tumour thrombus [416,417].
In two of the largest published studies a higher OS was different in patients with a level of thrombus in the renal vein and inferior caval vein and survival was also not associated with tumour size, grade, perinephric fat extension, sarcomatoid features, Eastern Cooperative Oncology Group PS and regional- and distant metastases in multivariate analysis [416,417]. Therefore, all patients with non-metastatic disease and venous tumour thrombus, and an acceptable PS, should be considered for surgical intervention, irrespective of the extent of tumour thrombus at presentation. The surgical technique (open vs. laparoscopic vs. robotic) and approach for each case should be selected based on the extent of tumour thrombus.
A systematic review was undertaken on the management of venous tumour thrombus in non-metastatic RCC, with a high risk of bias across all studies [418,419]. Minimal access techniques resulted in significantly shorter operating time compared with traditional median sternotomy [418]
The surgical method selected depended on the level of tumour thrombus and the grade of occlusion of the IVC [418]. The relative benefits and harms of other strategies and approaches regarding access to the IVC and the role of IVC filters and bypass procedures remain uncertain.
A systematic review comparing robot-assisted laparoscopic- and open tumour thrombectomy at all levels found lower transfusion rates and overall complication rates for the minimally-invasive approach, however major complication rates were similar to those in open thrombectomy. The optimal patient selection for the different approaches remains unclear [419].
A feasibility study with neoadjuvant axitinib to reduce the thrombus was positive, but larger studies are needed before its use can be routinely implemented into clinical practice [420].
7.2.4. Management of locally-advanced unresectable RCC
The management of locally-advanced unresectable RCC should be based around systemic therapy [421]. A multidisciplinary evaluation, including urologists, medical oncologists and radiation therapists is suggested to maximise cancer control, pain control and the best supportive care. In patients with non-resectable disease, embolisation can control symptoms, including visible haematuria or flank pain [298,299,422,423].
7.2.4.1. Summary of evidence and recommendations for lymph node dissection, the management of RCC with venous tumour thrombus and unresectable tumours
Summary of evidence | LE |
In patients with locally-advanced disease, the survival benefit of LN dissection is unproven but LN dissection has significant staging, prognosis, adjuvant therapy and follow-up implications. | 3 |
Low-quality data suggest that tumour thrombus excision in non-metastatic disease may be beneficial. | 3 |
Recommendations | Strength rating |
During nephrectomy, remove clinically enlarged lymph nodes for staging, prognosis and follow-up implications. | Weak |
Remove the renal tumour and thrombus in case of venous involvement in non-metastatic disease. | Strong |
Discuss treatment options in patients with locally-advanced unresectable RCC (biopsy and/or systemic therapy/deferred resection, or palliative management) within a multidisciplinary team to determine treatment goal. | Strong |
7.2.5. Neoadjuvant and adjuvant therapy
Neoadjuvant therapy is currently under investigation and available in clinical trials. There is currently no evidence from a systematic review (including ten retrospective studies and two RCTs) that adjuvant radiation therapy increases survival [424]. The impact on OS of adjuvant tumour vaccination in selected patients undergoing nephrectomy for T3 renal carcinomas remains unconfirmed [425-429] (LE: 1b). A similar observation was made in an adjuvant trial of girentuximab, a monoclonal antibody against carbonic anhydrase IX (CAIX) (ARISER Study) [430].
At present, there is no OS data supporting the use of adjuvant VEGFR or mTOR inhibitors. Thus far, several RCTs comparing VEGFR-TKI vs. placebo have been published [431-438]. Only S-TRAC, a trial of adjuvant sunitinib vs. placebo demonstrated a DFS benefit which was not reproduced in ASSURE, a trial of sunitinib and sorafenib vs. placebo. Due to an unfavourable AE profile and no survival advantage, none of these drugs are recommended [439].
7.2.5.1. PD-1 Inhibition: Keynote-564
The Keynote-564 trial is the first trial to report positive primary endpoint data on DFS [440,441]. Keynote-564 evaluated pembrolizumab (17 cycles of 3-weekly therapy) vs. placebo as adjuvant therapy in 994 patients with intermediate (pT2, grade 4 or sarcomatoid, N0, M0; or pT3, any grade, N0, M0) or high risk (pT4, any grade, N0, M0; or pT any stage, and grade, or N+, M0), or M1 (no evidence of disease [NED] after primary tumour plus soft tissue metastases completely resected < one year from nephrectomy) disease. The median follow-up, defined as time from randomisation to data cut-off, was 24.1 months. The primary endpoint of DFS per investigator assessment was significantly improved in the pembrolizumab group vs. placebo (HR: 0.68, 95% CI: 0.53–0.87, p = 0.001). The estimated 24-month DFS rate was 77% vs. 68% for pembrolizumab and placebo, respectively. Benefit occurred across broad subgroups of patients including those with M1/NED disease post-surgery (n = 58 [6%]). Investigator assessed DFS was considered preferable to DFS by central review due to its clinical applicability. Overall survival showed a non-statistically significant trend towards a benefit in the pembrolizumab arm (HR: 0.54, 95% CI: 0.30–0.96, p = 0.0164). Follow-up was short and few OS events occurred (2-year OS rate of 97% [pembrolizumab] vs. 94% [placebo]). Grade 3–5 all-cause AEs occurred in 32% vs. 18% of patients for pembrolizumab and placebo, respectively. Quality of life assessment by FKSI-DRS and QLQ30 did not show a statistically significant or clinically meaningful deterioration in health-related QoL or symptom scores for either adjuvant pembrolizumab or placebo.
7.2.5.2. PD-L1 inhibition: IMmotion010
The IMmotion010 phase III trial was the first adjuvant ICI trial to be developed in RCC to investigate the effect of a PD-L1 inhibitor on DFS [442]. IMmotion010 evaluated atezolizumab 1200 mg (once every 3 weeks for 16 cycles or one year) vs. placebo as adjuvant therapy in 778 patients with increased risk of recurrence defined as: pT2, grade 4 or sarcomatoid, N0, M0; or pT3, grade 3–4, N0, M0; or pT3b/c/T4, any grade, N0, M0; or pT any stage and grade, pN1, M0, or M1 no NED after primary tumour plus soft tissue metastases completely resected either synchronous or if metachronous, > 12 months from nephrectomy.
The minimum follow-up, defined as time from randomisation to data cut-off, was 38.6 months. The primary endpoint of DFS per investigator assessment was not met in the atezolizumab group vs. placebo (HR: 0.93, 95% CI: 0.75–1.15, p = 0.4950) with a median DFS of 57.2 months (95% CI: 44.6, NE) for atezolizumab vs. 49.5 months for placebo (47.4, NE). None of the exploratory subgroups suggested a DFS benefit with atezolizumab, most notably the M1 NED subgroup (n = 108/13.9%) which was larger than in Keynote-564 (5.8%), the sarcomatoid subgroup and the subgroup expressing > 1% PD-L1 had a HR of 0.93 (0.58–1.49), 0.77 (0.44–1.36) and 0.83 (0.63–1.10), respectively.
There were no OS differences. Grade 3–4 all-cause and treatment-related AEs occurred in 27.2% and 14.1% vs. 21.1% and 4.7% of patients for atezolizumab and placebo, respectively. There were no treatment-related grade 5 AEs.
7.2.5.3. PD-1 and CTLA-4 inhibition: CheckMate 914
CheckMate 914 was the first phase III trial to investigate a combination of nivolumab plus ipilimumab vs. placebo as adjuvant treatment in RCC (part A) [443]. Subsequently, a nivolumab monotherapy arm was also added to the trial (part B). The following results relate to part A which evaluated nivolumab 240 mg every two weeks (Q2W) for 12 cycles or 6 months plus ipilimumab 1 mg/kg Q6W for 4 cycles vs. placebo in 816 patients with recurrence risk defined as pT2a, grade 3 or 4, N0, M0; pT2b/T3/T4, any grade, N0, M0, or pT any stage, any grade, pN1, M0. The median time of follow-up, defined as time from randomisation to data cut-off, was 37 months. The primary endpoint of DFS per investigator assessment was not met in the nivolumab plus ipilimumab group vs. placebo (HR 0.92 [0.71–1.19], p = 0.5347). Of the exploratory subgroups, patients with sarcomatoid tumours (n = 40) and those with > 1% PD-L1 expression (n = 107) had a HR of 0.29 (0.09–0.91) and 0.46 (0.23–0.94) in favour of the ICI combination, respectively.
All-cause treatment discontinuation due to study drug occurred in 43% and 33% in the nivolumab plus ipilimumab group vs. 11% and 1% in the placebo group. Treatment-related AE grade > 3 were 29% in the nivolumab plus ipilimumab group and 2% in the placebo group with 4 deaths (1%) considered related to combination therapy. The high AE profile may have contributed to the lack of efficacy and patient retention. The results of the nivolumab arm are awaited.
7.2.5.4. Perioperative PD-1 inhibition: PROSPER
PROSPER is a peri-operative trial of neoadjuvant nivolumab (one cycle) followed by radical or partial nephrectomy and adjuvant nivolumab (480 mg IV q4 weeks) for nine doses compared to surgery followed by surveillance without a placebo [444]. Patients with clinical stage > T2 or T any N+ RCC or patients with selected oligometastatic disease were included if they had no evidence of disease within 12 weeks post-surgery. A total of 819 patients with clear cell (87%) and non-ccRCC were included, a biopsy in the nivolumab arm was mandatory. The primary endpoint of RFS was similar between the arms (HR: 0.97; 95% CI: 0.74–1.28; p = 0.43) and the trial was stopped by DSMC. The OS was not statistically different (HR: 1.48; 95% CI: 0.89–2.48; p = 0.93), although not mature. Grade 3–4 AEs occurred in 20% (nivolumab arm) and 6% (control arm) of patients, respectively. Fifteen (4%) patients died in the nivolumab arm and 18 (4%) in the surgery alone arm.
Following the application of the EAU Guidelines modified GRADE assessment the panel reached consensus and issued a weak recommendation for adjuvant pembrolizumab for patients with high-risk (defined as per study) operable ccRCC until final OS data are available [445]. This decision was taken as immune checkpoint inhibitor therapy has a different mode of action than VEGFR-TKI resulting in complete responses in up to 16% of patients in PD-1 unselected populations in metastatic disease [453]. Despite immature OS data with the early OS signal potentially driven by the M1 population the Panel cannot exclude that a survival benefit will emerge. This was not the case in the adjuvant sunitinib trial (STRAC) [443,446]. The Panel took the following evidence limitations into account when deciding to make to a weak recommendation for adjuvant pembrolizumab:
- A high proportion of patients, cured by surgery, are receiving unnecessary, and potentially harmful treatment.
- The tolerability profile is acceptable but grade 3–5 AEs were higher with 14.7% in the pembrolizumab arm vs. the placebo arm (occurring in approximately one-third of patients, all cause). Approximately 18% of patients required treatment discontinuation early for AEs which gives a broad indicator of tolerability. There is a significant risk of life-changing toxicity.
- Other ICI trials have not shown consistent results.
- Biomarker analysis to predict outcome and AEs are not available.
- Final OS data are not yet available.
The results of IMmotion010, CheckMate 914 and PROSPER need to be discussed with patients [442-444]. Meta-analysis with these data sets is not recommended due to heterogeneity across the ICI studies. It is likely that there are several reasons behind these inconsistent results, including study population with potential heterogeneity independent of TNM risk groups, selection criteria and trial design. To date pembrolizumab is the only positive trial [446].
While the results of IMmotion010 may reflect the non-significant OS results seen in the metastatic setting with PD-L1 inhibitors (IMmotion151, Javelin 101), the results of CheckMate 914 and PROSPER are more difficult to interpret. Nivolumab and ipilimumab leads to durable remission and long-term OS in metastatic disease and nivolumab has a similar mode of action as pembrolizumab (anti PD-1).
The high treatment discontinuation rate of 33% in CheckMate 914 is of concern and may have had an impact on the trial effectivity (20% in Keynote-564). The Panel strongly feels that biomarker work on all of these trials should occur to identify patients that do respond to therapy and to give a better explanation for the inconsistent results. Treatment of unselected patients in the adjuvant setting based on the Keynote-564 criteria will result in a large proportion of patients receiving unnecessary therapy. In the absence of OS data or appropriate biomarkers, the patient preference should be leading in a shared decision-making process. Patients considering adjuvant therapy should be aware of all trials and not be presented with only one data set.
Table 7.1: Overview phase III trials of PD-1 immune checkpoint inhibitors in adjuvant RCC
Phase III trial of PD-1 immune checkpoint inhibitors in adjuvant RCC | ||||||
Study | N | Experimental arm | Primary endpoint | Risk groups | DFS (mo) Median (95% CI) HR | OS (mo.) Median (95% CI) HR |
Keynote-564 NCT03142334 Median follow-up | 994 | PEMBRO 200 mg IV Q3W | DFS in the ITT by IR | Intermediate-high: pT2 grade 4 or sarcomatoid; pT3 any grade High: pT4 any grade, pN1 M1 NED: cM0 after resection of oligometastatic disease < 12 mo. | (ITT) PEMBRO: NR (NE) PLACEBO: NR (NE) HR: 0.63 (95% CI: 0.50–0.80) p < 0.002 DFS at 24 mo.: PEMBRO: 78.3% PLACEBO: 67.3% | (ITT) PEMBRO: NR (NE) PLACEBO: NR (NE) HR: 0.52 (95% not significant alive at 30 mo.: PEMBRO: 95.7% PLACEBO: 91.4% |
IMmotion010 NCT03024996 Median follow-up | 778 | ATEZO | DFS in the ITT by IR | By TNM: M1 NED: cM0 after resection of oligometastatic disease (synchronous or | (ITT) ATEZO: 57.2 PLACEBO: 49.5 (47.4–NE) HR: 0.93 (95% p = 0.4950 DFS at 24 mo.: NR | (ITT) ATEZO: PLACEBO: HR : 0.97 (95% alive at 24 mo.: NR |
CheckMate 914 NCT03138512 Median follow-up of 37.0 mo. [443] | 816 | NIVO 240 mg | DFS in the ITT | By TNM: pT2b/T3/T4 any grade, pN1 | (ITT) NIVO + IPI: NR (NE) PLACEBO: 50.7 (48.1–NE) HR: 0.92 (95% p = 0.5347 DFS at 24 mo.: NIVO + IPI: 76.4% PLACEBO: 74.0% | NR |
PROSPER NCT03055013 Median follow-up: NR [444] | 779 | Neoadjuvant NIVO 240 mg | RFS in the ITT by IR | By TNM: | (ITT), RFS: NIVO: NR (NE) Observation: NR (NE) HR: 0.97 (95% p = 0.43 | (ITT) NIVO: NR (NE) Observation: NR (NE) HR: 1.48 (95% p = 0.93 |
ATEZO = atezolizumab; BICR = blinded independent central review; CI = confidence interval; DFS = disease-freesurvival; HR = hazard ratio; IPI = ipilimumab; IR = investigator review; ITT = intention-to-treat; IV = intravenous; mo = months; NE = non-estimable; NED = no evidence of disease; NIVO = nivolumab; NR = not reached; OS = overall survival; PD-1 = programmed death-receptor 1; PEMBRO = pembrolizumab; PFS = progression-free survival; Q2W = every 2 weeks; Q3W = every 3 weeks.
7.2.5.5. Summary of evidence and recommendations for neoadjuvant and adjuvant therapy
Summary of evidence | LE |
Adjuvant sorafenib, pazopanib, everolimus, girentuximab, or axitinib does not improve DFS or OS after nephrectomy. | 1b |
In one single RCT, in selected high-risk patients, adjuvant sunitinib improved DFS but not OS. | 1b |
Adjuvant pembrolizumab defined by the inclusion criteria of the trial* after nephrectomy improves DFS. | 1b |
Adjuvant PD-L1 inhibition with atezolizumab did not improve DFS or OS. | 1b |
Adjuvant dual PD-1 and CTLA-4 inhibition with nivolumab and ipilimumab did not improve DFS. | 1b |
Peri-operative treatment with nivolumab did not improve RFS. | 1b |
The lack of biomarker data is hindering progress in this field. Adjuvant RCTs are ongoing to evaluate the benefit of adjuvant immunotherapy after nephrectomy in high-risk patients. | 4 |
* pT2 G4 or pT3 any G; pT4 any G; pN+ any G; M1, NED after resection of metastases.
Recommendations | Strength rating |
Discuss the contradictory results of the available adjuvant ICI trials with patients to facilitate shared decision making. | Strong |
Inform patients about the potential risk of overtreatment and immune-related side effects if adjuvant therapy is considered. | Strong |
Do not offer adjuvant therapy with sorafenib, pazopanib, everolimus, girentuximab, or axitinib. | Strong |
Do not offer adjuvant sunitinib following surgically resected high-risk clear-cell renal cell carcinoma (ccRCC). | Weak |
Offer adjuvant pembrolizumab to ccRCC patients, preferably within 12–16 weeks post-nephrectomy, with a recurrence risk as defined in the Keynote-564 trial: Intermediate-high risk:
High risk:
| Weak |
7.3. Advanced/metastatic RCC
7.3.1. Local therapy of advanced/metastatic RCC
7.3.1.1. Cytoreductive nephrectomy
Tumour resection is potentially curative only if all tumour deposits are excised. This includes patients with the primary tumour in place and single- or oligometastatic resectable disease. For most patients with metastatic disease, cytoreductive nephrectomy (CN) is palliative and systemic treatments are necessary. In a combined analysis of two RCTs comparing CN+ IFN-based immunotherapy vs. IFN-based immunotherapy only, increased long-term survival was found in patients treated with CN [447].
However, IFN-based immunotherapy is no longer relevant in contemporary clinical practice. In order to investigate the role and sequence of CN in the era of targeted therapy, a structured literature assessment was performed to identify relevant RCTs and systematic reviews published between July 1st - June 30th 2019.
Two RCTs [448,449] and a narrative systematic review were identified [450]. The narrative systematic review included both RCTs and 10 non-RCTs. CARMENA, a phase III non-inferiority RCT investigating immediate CN followed by sunitinib vs. sunitinib alone, showed that sunitinib alone was not inferior to CN followed by sunitinib with regard to OS [448]. The trial included 450 patients with metastatic ccRCC of intermediate- and MSKCC poor risk of whom 226 were randomised to immediate CN followed by sunitinib and 224 to sunitinib alone. Patients in both arms had a median of two metastatic sites. Patients in both arms had a tumour burden of a median/mean of 140 mL of measurable disease by Response Evaluation Criteria In Solid Tumours (RECIST) 1.1, of which 80 mL accounted for the primary tumour. The study did not reach the full accrual of 576 patients and the Independent Data Monitoring Commission (IDMC) advised the trial steering committee to close the study. In an ITT analysis after a median follow-up of 50.9 months, median OS with CN was 13.9 months vs. 18.4 months with sunitinib alone (HR: 0.89, 95% CI: 0.71–1.10). This was found in both risk groups. For MSKCC intermediate-risk patients (n = 256) median OS was 19.0 months with CN and 23.4 months with sunitinib alone (HR: 0.92, 95% CI: 0.60–1.24) and for MSKCC poor risk (n = 193) 10.2 months and 13.3 months, respectively (HR: 0.86, 95% CI: 0.62–1.17). Non-inferiority was also found in two per-protocol analyses accounting for patients in the CN arm who either did not undergo surgery (n = 16) or did not receive sunitinib (n = 40), and patients in the sunitinib-only arm who did not receive the study drug (n = 11). Median PFS in the ITT population was 7.2 months with CN and 8.3 months with sunitinib alone (HR: 0.82, 95% CI: 0.67–1.00). The clinical benefit rate, defined as disease control beyond twelve weeks was 36.6% with CN and 47.9% with sunitinib alone (p = 0.022). Of note, 38 patients in the sunitinib-only arm required secondary CN due to acute symptoms or for complete or near-complete response. The median time from randomisation to secondary CN was 11.1 months.
The randomised EORTC SURTIME study revealed that the sequence of CN and sunitinib did not affect PFS (HR: 0.88, 95% CI: 0.59–1.37, p = 0.569). The trial accrued poorly and therefore results are mainly exploratory. However, in secondary endpoint analysis a strong OS benefit was observed in favour of the deferred CN approach in the ITT population with a median OS of 32.4 (range 14.5–65.3) months in the deferred CN arm vs. 15.0 (9.3–29.5) months in the immediate CN arm (HR: 0.57, 95% CI: 0.34–0.95, p = 0.032). The deferred CN approach appears to select patients with inherent resistance to systemic therapy [449]. This confirms previous findings from single-arm phase II studies [450,451]. Moreover, deferred CN and surgery appear safe after sunitinib which supports the findings, with some caution, of the only available RCT. In patients with poor PS or IMDC poor risk, small primaries, and high metastatic volume and/or a sarcomatoid tumour, CN is not recommended [452]. These data are confirmed by CARMENA [448] and upfront pre-surgical VEGFR-targeted therapy followed by CN seems to be beneficial [453].
Meanwhile first-line therapy recommendations for patients with their primary tumour in place have changed to ICI combination therapy (see Section 7.4.2.4) with sunitinib and other VEGFR-TKI monotherapies reserved for those who cannot tolerate ICI combination or have no access to these drugs. High-level evidence regarding CN is not available for ICI combinations but up to 30% of patients with primary metastatic disease, treated with their tumour in place, were included in the pivotal ICI combination trials (Table 7.2). The subgroup HRs, where available, suggest better outcomes for the ICI combination compared to sunitinib monotherapy. In mRCC patients without a need for immediate drug treatment, a recent systematic review evaluating effects of CN demonstrated an OS advantage of CN [450]. These data were supported by a nation-wide registry study showing that patients selected for primary CN had a significant OS advantage across all age groups [454].
Table 7.2: Key trials on immune checkpoint inhibitor combinations for primary metastatic disease
Trial | Drug combination | Number and % of patients treated with primary tumour in place | Number of patients treated with the primary tumour in place | Subgroup analyses (HR with 95% CIs) | ||
ICI combination | sunitinib | PFS | OS | |||
CheckMate 214 [455] | ipilimumab + nivolumab | 187/847 (22%) | 84 | 103 | NA | 0.63 |
CheckMate 9ER [456] | cabozantinib + nivolumab | 196/651 (30.1%) | 101 | 95 | 0.63 | 0.79 |
Javelin 101 | axitinib + avelumab | 179/886 (20.2%) | 90 | 89 | 0.75 | NA |
KEYNOTE-426 [458] | axitinib + pembrolizumab | 143/861 (16.6%) | 73 | 70 | 0.68 | 0.57 |
CLEAR [459] | lenvatinib + pembrolizumab | 179/714 (25.1%) | 97 | 82 | 0.38 | 0.52 |
CI = confidence interval; HR = hazard ratio; ICI = immune checkpoint inhibitor; NA = not available;PFS = progression-free survival; OS = overall survival.
The results of CARMENA and SURTIME demonstrated that patients who require systemic therapy benefit from immediate drug treatment. While randomised trials to investigate deferred vs. no cytoreductive nephrectomy with ICI and ICI combinations are ongoing, the exploratory results from the ICI combination trials demonstrate that the respective IO + IO or TKI + IO combinations have a superior effect on the primary tumour and metastatic sites when compared to sunitinib alone (Table 7.2). In accordance with the CARMENA and SURTIME data this suggests that mRCC patients and IMDC intermediate- and poor-risk groups with their primary tumour in place should be treated with upfront IO-based combinations. In patients with a clinical response to IO-based combinations, a subsequent CN may be considered.
7.3.1.1.1. Embolisation of the primary tumour
In patients unfit for surgery or with non-resectable disease, embolisation can control symptoms including visible haematuria or flank pain [298,299,422] (see recommendations Section 7.1.2.2.4).
7.3.1.1.2. Summary of evidence and recommendations for local therapy of advanced/metastatic RCC
Summary of evidence | LE |
Deferred CN with pre-surgical sunitinib in intermediate-risk patients with cc-mRCC shows a survival benefit in secondary endpoint analyses and selects out patients with inherent resistance to systemic therapy. | 2b |
Sunitinib alone is non-inferior compared to immediate CN followed by sunitinib in patients with MSKCC intermediate and poor risk who require systemic therapy with VEGFR-TKI. | 1a |
Cytoreductive nephrectomy in patients with simultaneous complete resection of a single metastasis or oligometastases may improve survival and delay systemic therapy. | 3 |
Patients with MSKCC or IMDC poor risk do not benefit from CN. | 1a |
Patients with their primary tumour in place treated with IO-based combination therapy have better PFS and OS in exploratory subgroup analyses compared to treatment with sunitinib. | 2b |
Recommendations | Strength rating |
Do not perform cytoreductive nephrectomy (CN) in MSKCC poor-risk patients. | Strong |
Do not perform immediate CN in intermediate-risk patients who have an asymptomatic synchronous primary tumour and require systemic therapy. | Weak |
Start systemic therapy without CN in intermediate-risk patients who have an asymptomatic synchronous primary tumour and require systemic therapy. | Weak |
Discuss delayed CN with patients who derive clinical benefit from systemic therapy. | Weak |
Perform immediate CN in patients with a good performance status who do not require systemic therapy. | Weak |
Perform immediate CN in patients with oligometastases when complete local treatment of the metastases can be achieved. | Weak |
7.3.2. Local therapy of metastases in metastatic RCC
A systematic review of the local treatment of metastases from RCC in any organ was undertaken [460]. Interventions included metastasectomy, various radiotherapy modalities, and no local treatment. The outcomes assessed were OS, CSS and PFS, local symptom control and AEs. A risk-of-bias assessment was conducted [461]. Of the 2,235 studies identified only sixteen non-randomised comparative studies were included.
Eight studies reported on local therapies of RCC-metastases in various organs [462-470]. This included metastases to any single organ or multiple organs. Three studies reported on local therapies of RCC metastases in bone, including the spine [470-472], two in the brain [473,474] and one each in the liver [475] lung [476] and pancreas [477]. Three studies were published as abstracts only [465,467,476]. Data were too heterogeneous to meta-analyse. There was considerable variation in the type and distribution of systemic therapies (cytokines and VEGF-inhibitors) and in reporting the results.
7.3.2.1. Complete versus no/incomplete metastasectomy
A systematic review, including only eight studies, compared complete vs. no and/or incomplete metastasectomy of RCC metastases in various organs [462-469]. In one study complete resection was achieved in only 45% of the metastasectomy cohort, which was compared with no metastasectomy [469]. Non-surgical modalities were not applied. Six studies [463-465,467-469] reported a significantly longer median OS or CSS following complete metastasectomy (the median value for OS or CSS was 40.75 months, range 23–122 months) compared with incomplete and/or no metastasectomy (the median value for OS or CSS was 14.8 months, range 8.4–55.5 months). Of the two remaining studies, one [462] showed no significant difference in CSS between complete and no metastasectomy, and one [466] reported a longer median OS for metastasectomy albeit no p-value was provided.
Three studies reported on treatment of RCC metastases in the lung [476], liver [475], and pancreas [477], respectively. The lung study reported a significantly higher median OS for metastasectomy vs. medical therapy only for both targeted therapy and immunotherapy. Similarly, the liver and pancreas study reported a significantly higher median OS and 5-year OS for metastasectomy vs. no metastasectomy.
More recently, a prospective study evaluated US-guided endoscopic RFA in patients with pancreatic metastases (n = 12). Median size of a single pancreatic metastasis was 17 mm. After 27.7 months of follow-up, the 6- and 12-month focal control rates were 84% and 73%, respectively, although two severe complications occurred. Due to the low numbers of patients in this study, RFA for pancreatic metastases will still remain experimental [478].
7.3.2.2. Local therapies for RCC bone metastases
Of the three studies identified, one compared single-dose image-guided radiotherapy (IGRT) with hypofractionated IGRT in patients with RCC bone metastases [472]. Single-dose IGRT (> 24 Gy) had a significantly better 3-year actuarial local PFS rate, also shown by Cox regression analysis. Another study compared metastasectomy/curettage and local stabilisation with no surgery of solitary RCC bone metastases in various locations [470]. A significantly higher 5-year CSS rate was observed in the intervention group. After adjusting for prior nephrectomy, gender and age, multivariable analysis still favoured metastasectomy/curettage and stabilisation. A third study compared the efficacy and durability of pain relief between single-dose stereotactic body radiotherapy (SBRT) and conventional radiotherapy in patients with RCC bone metastases to the spine [471]. Pain, ORR, time-to-pain relief and duration of pain relief were similar.
7.3.2.3. Local therapies for RCC brain metastases
Two studies on RCC brain metastases were included. A 3-arm study compared stereotactic radiosurgery (SRS) vs. whole brain radiotherapy (WBRT) vs. SRS and WBRT [473]. Each group was further subdivided into recursive partitioning analysis (RPA) classes I to III (I favourable, II moderate and III poor patient status). Two-year OS and intra-cerebral control were equivalent in patients treated with SRS alone and SRS plus WBRT.
Both treatments were superior to WBRT alone in the general study population and in the RPA subgroup analyses. A comparison of SRS vs. SRS and WBRT in a subgroup analysis of RPA class I showed significantly better 2-year OS and intra-cerebral control for SRS plus WBRT based on only three participants. The other study compared fractionated stereotactic radiotherapy (FSRT) with metastasectomy and conventional radiotherapy or conventional radiotherapy alone [474]. Several patients in all groups underwent alternative surgical and non-surgical treatments after initial treatment. One-, two- and 3-year survival rates were higher but not significantly so for FSRT as for metastasectomy and conventional radiotherapy, or conventional radiotherapy alone. Fractionated stereotactic radiotherapy did not result in a significantly better 2-year local control rate compared with metastasectomy plus conventional radiotherapy.
Stereotactic radiotherapy (SRT) with a median physical dose of 20 (18–30) Gy and a biologically effective dose (DED10) of 63.3 (45–125) Gy in a median (range) of 1 (1–6) fractions for 1–5 brain metastases were safe also during ICI and targeted therapy [479]. Targeted therapy was paused only in one-third of patients for 2–21 days. Local control at all sites, including extracranial, was 75% at one year. After one year, 62% of patients remained on the same systemic therapy as at the time of SRT, which was more frequent for ICI therapy as compared to targeted therapy (83% vs. 36%; p = 0.035). No grade 4 or 5 toxicity was observed.
7.3.2.4. Embolisation of metastases
Embolisation prior to resection of hypervascular bone or spinal metastases can reduce intra-operative blood loss [170]. In selected patients with painful bone or paravertebral metastases, embolisation can relieve symptoms [171] (see recommendation Section 7.1.2.2.4).
7.3.2.5. Stereotactic radiotherapy in oligo-recurrent and oligo-progressive metastases
Retrospective analysis of 207 patients with oligo-recurrent and oligo-progressive lesions in mainly bones and lungs with or without systemic therapy (mainly targeted therapy) demonstrated 2-year local control rate of 78.3% (95% CI: 72.5–83.0). 1, 2 and 3-year local control rates were 89.4%, 80.1% and 76.6% in oligo-recurrent patients, and 82.7%, 76.9% and 64.3% in those with oligo-progressive disease, respectively. Median applied biologically effective dose (BED)10 was 60 Gy. Median time to subsequent systemic therapy was 13.9 months and median PFS was 37.9 months. No grade 3 or higher toxicities were reported [480].
Similar results in oligo-progressive mRCC has been reported in a prospective study including 37 patients with IMDC favourable- and intermediate risk where 1-year local control of the irradiated lesions was 93% (95% CI: 71–98%) and median time to change in systemic TKI therapy was 12.6 months (95% CI: 9.6–17.4 months). Median therapy prior to study entry was 18.6 months and therapy was discontinued during SRT. The median BED10 was 72 Gy, corresponding to a SRT dose of 40 Gy in 5 fractions. Median PFS was 9.3 months and there were no reported grade 3 acute or late toxicities [481].
7.3.2.6. Adjuvant treatment in cM0 patients after metastasectomy
Patients after metastasectomy and no evidence of disease (cM0) have a high risk of relapse. Recent attempts to reduce RFS by offering adjuvant TKI treatment after metastasectomy did not demonstrate an improvement in RFS. In a recent phase II trial 129 patients were randomised to either pazopanib 800 mg daily vs. placebo for 52 weeks. The primary study endpoint of a 42% DFS improvement from 25% to 45% at three years was not met. Hazard ratio for DFS in pazopanib vs. placebo-treated patients was 0.85 (0.55–1.31), p = 0.47 [172]. A second phase II trial randomised 69 ccRCC patients after metastasectomy and no evidence of disease to either sorafenib (400 mg twice daily) or observation. The study was terminated early due to slow accrual and the availability of new agents and multimodal treatment options, including surgery or a locoregional approach. The primary endpoint of RFS was not reached with a RFS of 21 months in the sorafenib arms vs. 37 months in the observation arm (p = 0.404) [173], which also did not change after a longer median follow-up period of 42 months [173].
KEYNOTE-564 included a small percentage of patients who were treated by nephrectomy and complete metastasectomy within one year after primary diagnosis (6% in the experimental arm and 6% in the placebo arm) [440,441]. A metachronous interval of < 1 year for recurrences following surgery with curative intent is a poor prognostic factor by IMDC classification [256,482]. Systemic therapy based on immune combinations has stronger levels of evidence than surgery in this intermediate/advanced disease setting [483]. Also, TKI-driven adjuvant trials after metastasectomy have shown no DFS or OS benefit [172,173].
Results for single-agent pembrolizumab post-surgery for metastatic disease are therefore difficult to interpret due to the small subgroup. Nevertheless, the DFS HR of 0.29 (95% CI: 0.12–0.69) in favour of resection of M1 to NED plus pembrolizumab shows that patients with subclinical, but progressive, disease who were subjected to metastasectomy had a benefit of adjuvant systemic therapy with pembrolizumab. Based on the current data it cannot be concluded that for patients with oligo-progressive disease, metastasectomy within the first year of initial diagnosis of the primary and subsequent adjuvant pembrolizumab is superior to a period of observation and dual IO-based combination first-line therapy upon progression. Data from the TKI era suggest that patients with oligometastatic disease recurrence can be observed for up to a median of sixteen months before systemic therapy is required and that this practice is common in real-world settings (30%) [484,485].
In addition, it is possible that metastasectomy may lead to poorer outcomes compared to systemic therapy approaches as a relapse within the first twelve months and presentation with synchronous (oligo- metastatic disease is attributed to the IMDC intermediate risk-group. The Panel therefore does not encourage metastasectomy and adjuvant pembrolizumab in this population with recurrent disease within one year after primary surgery. A careful reassessment of disease status to rule out rapid progressive disease should be performed. Data from another adjuvant ICI study with the PD-L1 inhibitor atezolizumab (IMmotion010) also included an M1 NED subgroup which showed no DFS advantage [442]. This result underscores the need for caution in the treatment of the M1 NED subgroup.
7.3.2.7. Summary of evidence and recommendations for local therapy of metastases in metastatic RCC
Summary of evidence | LE |
Retrospective comparative studies consistently point towards a benefit of complete metastasectomy in mRCC patients in terms of OS, CSS and delay of systemic therapy. | 3 |
A single-arm prospective and retrospective study support that oligometastases can be observed for up to 16 months before systemic therapy is required due to progression. | 2a |
Radiotherapy to bone and brain metastases from RCC can induce significant relief from local symptoms (e.g., pain). | 3 |
Tyrosine kinase inhibitors treatment after metastasectomy in patients with no evidence of disease did not improve RFS when compared to placebo or observation. | 1b |
Recommendations | Strength rating |
To control local symptoms, offer ablative therapy, including metastasectomy, to patients with metastatic disease and favourable disease factors and in whom complete resection is achievable. | Weak |
Offer stereotactic radiotherapy for clinically relevant bone- or brain metastases for local control and symptom relief. | Weak |
Do not offer tyrosine kinase inhibitor treatment to mRCC patients after metastasectomy and no evidence of disease. | Strong |
Perform a confirmatory axial scan of disease status prior to metastasectomy to rule out rapid progressive metastatic disease which requires systemic treatment. | Weak |
Before initiating systemic therapy for oligometastases that cannot be resected, discuss with your patient a period of observation until progression is confirmed. | Weak |
7.4. Systemic therapy for advanced/metastatic RCC
7.4.1. Chemotherapy
Chemotherapy has proven to be generally ineffective in the treatment of RCC but can be offered to patients with collecting duct or medullary carcinoma [174].
7.4.1.1. Recommendation for systemic therapy in advanced/metastatic RCC
Recommendation | Strength rating |
Do not offer chemotherapy to patients with metastatic renal cell carcinoma. | Strong |
7.4.2. Targeted therapies
In sporadic ccRCC, HIF accumulation due to VHL-inactivation results in overexpression of VEGF and platelet-derived growth factor (PDGF), which promote neo-angiogenesis [486-488]. This process substantially contributes to the development and progression of RCC. Several targeting drugs for the treatment of mRCC are approved in both the USA and Europe.
Most published trials have selected for clear-cell carcinoma subtypes, thus no robust evidence-based recommendations can be given for non-ccRCC subtypes.
In major trials leading to registration of the approved targeted agents, patients were stratified according to the IMDC risk model (Table 7.3) [258].
Table 7.3: Median OS and percentage of patients surviving two years treated in the era of targeted therapy per IMDC risk group*#
Median OS and percentage of patients surviving two years treated in the era of targeted therapy per IMDC risk group | ||||
IMDC Model | Patients# | Median OS* (months) | 2-yr OS (95% CI)# | |
n | % | |||
Favourable | 157 | 18 | 43.2 | 75% (65–82%) |
Intermediate | 440 | 52 | 22.5 | 53% (46–59%) |
Poor | 252 | 30 | 7.8 | 7% (2–16%) |
* Based on # based on [258, 482]
CI = confidence interval; IMDC = Metastatic Renal Cancer Database Consortium; n = number of patients;OS = overall survival; yr = year.
7.4.2.1. Tyrosine kinase inhibitors
7.4.2.1.1. Sunitinib
Sunitinib is an oral TKI inhibitor and has anti-tumour and anti-angiogenic activity. First-line monotherapy with sunitinib demonstrated significantly longer PFS compared with IFN-α. Overall survival was greater in patients treated with sunitinib (26.4 months) vs. IFN-α (21.8 months) despite crossover [489].
In the EFFECT trial, sunitinib 50 mg/day (four weeks on/two weeks off) was compared with continuous uninterrupted sunitinib 37.5 mg/day in patients with cc-mRCC [490]. No significant differences in OS were seen (23.1 vs. 23.5 months, p = 0.615). Toxicity was comparable in both arms. Because of the non-significant, but numerically longer time to progression with the standard 50 mg dosage, the authors recommended using this regimen. Alternate scheduling of sunitinib (two weeks on/one week off) is being used to manage toxicity, but robust data to support its use is lacking [491,492].
7.4.2.1.2. Pazopanib
Pazopanib is an oral angiogenesis inhibitor. In a trial of pazopanib vs. placebo in treatment-naive mRCC patients and cytokine-treated patients, a significant improvement in PFS and tumour response was observed [493].
A non-inferiority trial comparing pazopanib with sunitinib (COMPARZ) established pazopanib as an alternative to sunitinib. It showed that pazopanib was not associated with significantly worse PFS or OS compared to sunitinib. The two drugs had different toxicity profiles, and QoL was better with pazopanib [494]. In another patient-preference study (PISCES), patients preferred pazopanib to sunitinib (70% vs. 22%, p < 0.05) due to symptomatic toxicity [495]. Both studies were limited in that intermittent therapy (sunitinib) was compared with continuous therapy (pazopanib).
7.4.2.1.3. Axitinib
Axitinib is an oral selective second-generation inhibitor of VEGFR-1, -2, and -3. Axitinib was first evaluated as second-line treatment. In the AXIS trial, axitinib was compared to sorafenib in patients who had previously failed cytokine treatment or targeted agents (mainly sunitinib) [496].
The overall median PFS was greater for axitinib than sorafenib. Axitinib was associated with a greater PFS than sorafenib (4.8 vs. 3.4 months) after progression on sunitinib. Axitinib showed grade 3 diarrhoea in 11%, hypertension in 16%, and fatigue in 11% of patients. Final analysis of OS showed no significant differences between axitinib or sorafenib [497]. In a randomised phase III trial of axitinib vs. sorafenib in first-line treatment-naive cc-mRCC, a significant difference in median PFS between the treatment groups was not demonstrated, although the study was underpowered, raising the possibility of a type II error [498]. As a result of this study, axitinib is not approved for first-line therapy.
7.4.2.1.4. Cabozantinib
Cabozantinib is an oral inhibitor of tyrosine kinase, including MET, VEGF and AXL. Cabozantinib was investigated in a phase I study in patients resistant to VEGFR and mTOR inhibitors demonstrating objective responses and disease control [226]. Based on these results an RCT investigated cabozantinib vs. everolimus in patients with ccRCC failing one or more VEGF-targeted therapies (METEOR) [499,500]. Cabozantinib delayed PFS compared to everolimus in VEGF-targeted therapy refractory disease (HR: 0.58, 95% CI: 0.45–0.75) [499] (LE: 1b). The median OS was 21.4 months (95% CI: 18.7 to not estimable) with cabozantinib and 16.5 months (95% CI: 14.7–18.8) with everolimus in VEGF-resistant RCC. The HR for death was 0.66 (95% CI: 0.53–0.83, p = 0.0003) [500]. Grade 3 or 4 AEs were reported in 74% with cabozantinib and 65% with everolimus. Adverse events were managed with dose reductions; doses were reduced in 60% of the patients who received cabozantinib.
The Alliance A031203 CABOSUN randomised phase II trial comparing cabozantinib and sunitinib in first-line in 157 intermediate- and poor-risk patients favoured cabozantinib for RR and PFS, but not OS [501,502]. Cabozantinib significantly increased median PFS (8.2 vs. 5.6 months, adjusted HR: 0.66, 95% CI: 0.46 to 0.95; one-sided p = 0.012). Objective response rate was 46% (95% CI: 34–57) for cabozantinib vs. 18% (95% CI: 10–28) for sunitinib. All-causality grade 3 or 4 AEs were similar for cabozantinib and sunitinib. No difference in OS was seen. Due to limitations of the statistical analyses within this trial, the evidence is inferior over existing choices.
7.4.2.1.5. Lenvatinib
Lenvatinib is an oral multi-target TKI of VEGFR1, VEGFR2, and VEGFR3, with inhibitory activity against fibroblast growth factor receptors (FGFR1, FGFR2, FGFR3, and FGFR4), platelet growth factor receptor (PDGFRα), re-arranged during transfection (RET) and receptor for stem cell factor (KIT). It has recently been investigated in a randomised phase II study in combination with everolimus vs. lenvatinib or everolimus alone (see Section 7.4.4.1.1 for discussion of results) [503].
7.4.2.1.6. Tivozanib
Tivozanib is a potent and selective TKI of VEGFR1, VEGFR2, and VEGFR3 and was compared in two phase III trials with sorafenib in patients with mRCC [504,505]. Tivozanib was approved by the EMA in front-line mRCC. While it was associated with a PFS advantage in both studies, no OS advantage was seen. In view of the choice of sorafenib as the control arm in the front-line trial, the Panel considers there is too much uncertainty, and too many attractive alternatives, to support its use in this front-line setting.
7.4.2.2. Monoclonal VEGF antibody
Bevacizumab is a humanised monoclonal antibody. Initial first-line treatment in combination with IFN-α has been superseded by more effective therapies [506-508]. Bevacizumab in combination with atezolizumab has not been approved for treatment of mRCC (see Section 7.4.3.2) [509].
7.4.2.3.mTOR inhibitors
7.4.2.3.1. Temsirolimus
Temsirolimus is a specific inhibitor of mTOR [510]. Its use has been superseded as front-line treatment option.
7.4.2.3.2. Everolimus
Everolimus is an oral mTOR inhibitor, which is established in the treatment of VEGF-refractory disease. The RECORD-1 study compared everolimus plus best supportive care (BSC) vs. placebo plus BSC in patients with previously failed anti-VEGFR treatment (or previously intolerant of VEGF-targeted therapy) [511]. The data showed a median PFS of 4 vs. 1.9 months for everolimus and placebo, respectively [511].
The Panel consider, even in the absence of conclusive data, that everolimus may present a therapeutic option in patients who were intolerant to, or previously failed, immune- and VEGFR-targeted therapies (LE: 4). Recent phase II data suggest adding lenvatinib is attractive.
7.4.2.4. Summary of evidence and recommendations for single-agent targeted therapy in metastatic clear-cell RCC
Summary of evidence | LE |
Single-agent VEGF-targeted therapy has been superseded by immune checkpoint-based combination therapy. | 1b |
Pazopanib is non-inferior to sunitinib as first-line management option in mRCC. | 1b |
Cabozantinib in intermediate- and poor-risk treatment-naive ccRCC leads to better response rates and PFS but not OS when compared to sunitinib. | 2b |
Tivozanib has been EMA approved, but the evidence is still considered inferior over existing choices in the first-line setting. | 3 |
Single-agent VEGF-targeted therapies are preferentially recommended after first-line PD-L1-based combinations. Re-challenge with treatments already used should be avoided. | 3 |
Single-agent cabozantinib or nivolumab are superior to everolimus after one or more lines of VEGF-targeted therapy. | 1b |
Everolimus prolongs PFS after VEGF-targeted therapy when compared to placebo. This is no longer widely recommended before third-line therapy. | 1b |
Lenvatinib in combination with everolimus improved PFS over everolimus alone in VEGF-refractory disease. Its role after ICIs is uncertain. There is a lack of robust data on this combination making its recommendation challenging. | 2a |
Recommendations | Strength rating |
Offer nivolumab or cabozantinib for immune checkpoint inhibitor-naive vascular endothelial growth factor receptor (VEGFR)-refractory clear-cell metastatic renal cell carcinoma (cc-mRCC) after one or two lines of therapy. | Strong |
Sequencing the agent not used as second-line therapy (nivolumab or cabozantinib) for third-line therapy is recommended. | Weak |
Offer VEGF-tyrosine kinase inhibitors as second-line therapy to patients refractory to nivolumab plus ipilimumab or axitinib plus pembrolizumab or cabozantinib plus nivolumab or lenvatinib plus pembrolizumab. | Weak |
Offer cabozantinib after VEGF-targeted therapy in cc-mRCC. | Strong |
Sequence systemic therapy in treating mRCC. | Strong |
Offer immune checkpoint inhibitor combination therapy for advanced cc-mRCC with sarcomatoid features. | Weak |
7.4.3. Immunotherapy
7.4.3.1. Immune checkpoint inhibitors
7.4.3.1.1. Immuno-oncology monotherapy
Immune checkpoint inhibitor with monoclonal antibodies targets and blocks the inhibitory T-cell receptor PD-1 or cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4)-signalling to restore tumour-specific T-cell immunity [512]. Immune checkpoint inhibitor monotherapy has been investigated as second- and third-line therapy. A phase III trial of nivolumab vs. everolimus after one or two lines of VEGF-targeted therapy for mRCC with a clear cell component (CheckMate 025, NCT01668784) reported a longer OS, better QoL and fewer grade 3 or 4 AEs with nivolumab than with everolimus [513]. Nivolumab has superior OS to everolimus (HR: 0.73, 95% CI: 0.57–0.93, p < 0.002) in VEGF-refractory RCC with a median OS of 25 months for nivolumab and 19.6 months for everolimus with a 5-year OS probability of 26% vs. 18% [514] (LE: 1b). Patients who had failed multiple lines of VEGF-targeted therapy were included in this trial making the results broadly applicable. The trial included 15% MSKCC poor-risk patients. There was no PFS advantage with nivolumab despite the OS advantage. Progression-free survival does not appear to be a reliable surrogate of outcome for PD-1 therapy in RCC. Currently PD-L1 biomarkers are not used to select patients for this therapy.
There are no RCTs supporting the use of single-agent ICI in treatment-naive patients. Randomised phase II data for atezolizumab vs. sunitinib showed a HR of 1.19 (95% CI: 0.82–1.71) which did not justify further assessment of atezolizumab as single agent as first-line treatment option in this group of patients, despite high complete response rates in the biomarker-positive population [515]. Single-arm phase II data for pembrolizumab from the KEYNOTE-427 trial show high response rates of 38% (up to 50% in PD-L1+ patients), but a PFS of 8.7 months (95% CI: 6.7–12.2) [516]. Based on these results and in the absence of randomised phase III data, single-agent checkpoint inhibitor therapy is not recommended as an alternative in a first-line therapy setting.
In addition, several trials explored the strategy of nivolumab monotherapy in first-line ccRCC followed by a salvage strategy with nivolumab plus ipilimumab upon progression or if stable disease was the best response. Trial results do not support such a strategy which was frequently not feasible and of limited benefit [517,518].
7.4.3.2. Immunotherapy/combination therapy
The phase III trial CheckMate 214 (NCT 02231749) showed a superiority of nivolumab and ipilimumab over sunitinib. The primary endpoint population focused on the IMDC intermediate- and poor-risk population where the combination demonstrated an OS benefit (HR: 0.63, 95% CI: 0.44–0.89) which led to regulatory approval [455] and a paradigm shift in the treatment of mRCC [519]. Results from CheckMate 214 further established that the combination of ipilimumab and nivolumab was associated with higher response rates (RR) (39% in the ITT population), complete response rates (8% in the ITT population [central radiology review]) and duration of response compared to sunitinib. Progression-free survival did not achieve the pre-defined endpoint. The exploratory analysis of OS data in the PD-L1-positive population was 0.45 (95% CI: 0.29–0.41).
A recent update with 60-month data shows ongoing benefits for the immune combination with independently assessed complete response rates of 11% and a HR for OS in the IMDC intermediate- and poor-risk group of 0.68 (0.58–0.81) [520]. However, this complete response rate has not been consistent across trials for this combination (the Cosmic313 study showed complete response rates of 3% [521]).
In CheckMate 214 the 60-months OS probability was 43% for ipilimumab plus nivolumab vs. 31% for sunitinib, respectively [522]. In this update the IMDC good-risk group did not continue to perform better with sunitinib although this effect occurs due to a late overlap of the Kaplan-Meier curves (HR for OS: 0.94 [95% CI: 0.65–1.37]) [522]. Nivolumab plus ipilimumab was associated with 46% grade 3–4 toxicity and 1.5% treatment-related deaths. It should therefore be administered in centres with experience of immune combination therapy and appropriate supportive care within the context of a multidisciplinary team (LE: 4). PD-L1 biomarker is currently not used to select patients for therapy.
The frequency of steroid use has generated controversy and further analysis, as well as real world data, are required. For these reasons the Panel continues to recommend ipilimumab and nivolumab in the intermediate- and poor-risk population.
The KEYNOTE-426 trial (NCT02853331 reported results for the combination of axitinib plus pembrolizumab vs. sunitinib in 861 treatment-naive cc-mRCC patients [523]. Overall survival and PFS assessed by central independent review in the ITT population were the co-primary endpoints. Response rates and assessment in the PD-L1-positive patient population were secondary endpoints. With a minimum follow-up of 35.6 months
(median 42.8 months) this trial demonstrated an ongoing OS benefit for axitinib plus pembrolizumab in the ITT population (HR: 0.73, 95% CI: 0.60–0.88, p < 0.001). Median OS for axitinib plus pembrolizumab was 45.7 months (95% CI: 43.6 – NR) vs. 40.1 month (95% CI: 34.3 – 44.2) for sunitinib with a PFS benefit
(HR: 0.68, 95% CI: 0.58–0.80, p < 0.0001) which was shown across all IMDC subgroups for PFS, while OS was similar between axitinib plus pembrolizumab vs. sunitinib in the favourable subgroup with an OS benefit in the IMDC intermediate- and poor-risk groups. The complete response rate by independent review was 10% in the pembrolizumab plus axitinib arm and 4% in the sunitinib arm [524]. Treatment-related AEs (> grade 3) occurred in 63% of patients receiving axitinib and pembrolizumab vs. 58% of patients receiving sunitinib. Treatment-related deaths occurred in approximately 1% in both arms [523].
The phase III CheckMate 9ER trial randomised 651 patients to nivolumab plus cabozantinib (n = 323) or vs. sunitinib (n = 328) in treatment-naive cc-mRCC patients [456]. The primary endpoint of PFS assessed by central independent review in the ITT population was significantly prolonged for nivolumab plus cabozantinib (16.6 months) vs. sunitinib (8.3 months, HR: 0.51, 95% CI: 0.41–0.64, p < 0.0001). The nivolumab/cabozantinib combination also demonstrated a significant OS benefit in the secondary endpoint compared with sunitinib (HR: 0.60, CI: 0.40–0.89, p = 0.0010) after a median follow-up of 18.1 months in the initial report [525]. The independently assessed ORR was 55.7% vs. 27.1% with a complete response rate of 8% for nivolumab plus cabozantinib vs. 4.6% with sunitinib. The efficacy was observed independent of IMDC group and PD-L1 status. Treatment-related AEs (> grade 3) occurred in 61% of patients receiving cabozantinib and nivolumab vs. 51% of patients receiving sunitinib. Treatment-related deaths occurred in one patient in the nivolumab/cabozantinib arm and in two patients in the sunitinib arm. With an extended follow-up with median 32.9 months the median OS was 37.7 months in the nivolumab plus cabozantinib patients vs. 34.3 months (29.0–not estimable) in the sunitinib treated patients (HR: 0.70 [95% CI: 0.55–0.90, p = 0.0043). The updated median PFS was 16.6 months (12.8–19.8) vs. 8.3 months (7.0–9.7; HR 0.56 [95% CI: 0.4–0.68], p < 0·0001 [526].
The randomised phase III trial CLEAR (Lenvatinib/Everolimus or Lenvatinib/Pembrolizumab Versus Sunitinib Alone as Treatment of Advanced Renal Cell Carcinoma) was published [527]. CLEAR randomised a total of 1,069 patients (in a 1:1:1 ratio) to lenvatinib plus pembrolizumab (n = 355) vs. lenvatinib plus everolimus (n = 357) vs. sunitinib (n = 357). The trial reached its primary endpoint of independently assessed PFS at a median of 23.9 vs. 9.2 months, for lenvatinib plus pembrolizumab vs. sunitinib, respectively (HR: 0.39, 95% CI: 0.32–0.49, p < 0.001). Overall survival significantly improved with lenvatinib plus pembrolizumab vs. sunitinib (HR: 0.66, 95% CI: 0.49–0.88, p = 0.005). Objective response for lenvatinib plus pembrolizumab was 71% with 16% of the patients having a complete remission. Efficacy was observed across all IMDC risk groups, independently of PD-L1 status. Treatment-related AEs of grade 3 and higher with lenvatinib plus pembrolizumab were 72%. Treatment-related death occurred in four patients in the lenvatinib plus pembrolizumab arm and in one patient in the sunitinib arm.
The JAVELIN trial investigated 886 patients in a phase III RCT of avelumab plus axitinib vs. sunitinib [457]. The trial met one of its co-primary endpoints (PFS in the PD-L1-positive population at first interim analysis [median follow up 11.5 months]). Hazard ratios for PFS and OS in the ITT population were 0.69 (95% CI: 0.56–0.84) and 0.78 (95% CI: 0.55–1.08), respectively, but with a missing significant OS improvement also with longer follow-up [528]. The same applies to the atezolizumab/bevacizumab combination (IMmotion151) which also achieved a PFS advantage over sunitinib in the PD-L1-positive population at interim analysis and ITT (HR: 0.74, 95% CI: 0.57–0.96), but has not shown a significant OS advantage at final analysis (HR: 0.91 [95% CI: 0.76–1.08], p = 0.27) [509,529]. Therefore, these combinations cannot currently be recommended.
The COSMIC-313 trial is the first RCT to evaluate a triple combination of cabozantinib (40 mg) plus nivolumab plus ipilimumab vs. nivolumab plus ipilimumab, a current standard of care, in 855 patients with IMDC intermediate- and poor-risk [521]. The primary endpoint of PFS improvement, measured in a PFS ITT of 550 patient was met after 249 events occurred with a HR 0.73 (95% CI: 0.57–0.94, p = 0.013) favouring the triplet therapy. Median PFS was not reached (14.0–NE) vs. 11.3 months (7.7–18.2) in the control arm with a median follow-up of 20.2 months. Overall survival has yet to be reported. Objective response was 43% vs. 36% in the triplet vs. the control arm with a complete response rate of 3% in both arms. Treatment-related AEs of > grade 3 with cabozantinib plus nivolumab plus ipilimumab were 73% vs. 41% in the nivolumab plus ipilimumab control arm. The use of high-dose steroids (> = 40 mg prednisolone or equivalent) was 58% (triplet) vs. 35%. (control). Treatment discontinuation rate of any agent was high in the triplet arm (45%) compared to the doublet (24%), whilst discontinuation of all treatments due to the same AE was 12% vs. 5% in the control arm.
Although the primary endpoint of PFS was met, objective response rates of the triplet combination are modest as known for TKI + IO doublets. Treatment-related AEs are high with a high rate of treatment discontinuation. As the OS rate is currently unknown, the additional benefit of this triplet therapy compared to standard immune-based doublet therapy is still uncertain.
Table 7.4: First line immune checkpoint inhibitor combination trials for clear-cell RCC
Cross trial comparison is not recommended and should occur with caution
Study | N | Experimental arm | Primary endpoint | Risk groups | PFS (mo) Median (95% CI) HR | OS (mo) Median (95% CI) HR |
KEYNOTE-426 NCT02853331 Median follow-up 42.8 months | 861 | PEMBRO vs. SUN 50 mg | PFS and OS in the ITT by BICR | IMDC FAV 31% IMD 56% POOR 13% MSKCC Not determined | (ITT) PEMBRO + AXI: 15.7 (13.6–20.2) SUN: 11.1 (8.9–12.5) HR: 0.68 (95% CI: 0.58–0.80) p < 0.0001 | (ITT) PEMBRO + AXI: 45.7 (43.6–NR) SUN: 40.1 HR: 0.73 (95% CI: 0.60–0.88) p = 0.001 |
JAVELIN 101 NCT02684006 Median follow-up 19 months | 886 | AVE 10 mg/kg vs. SUN 50 mg | PFS in the PD-L1+ population and OS in the ITT by BICR | IMDC FAV 22% IMD 62% POOR 16% MSKCC FAV 23% IMD 66% POOR 12% | (PD-L1+) AVE + AXI: 13.8 (10.1–20.7) SUN: 7.0 (5.7–9.6) HR: 0.62 (95% CI: 0.49–0.78) p < 0.0001 | (PD-L1+) AVE + AXI: NR SUN: 28.6 (27.4–NE) HR: 0.83 (95% CI: 0.60–1.15) p = 0.1301 |
IMmotion151 NCT02420821 Median follow-up 24 months | 915 | ATEZO vs. SUN 50 mg PO QD 4/2 wk | PFS in the PD-L1+ population and OS in the ITT by IR | IMDC Not determined MSKCC FAV 20% IMD 69% POOR 12% | (PD-L1+) ATEZO + BEV: 11.2 (8.9–15.0) SUN: 7.7 (6.8–9.7) HR: 0.74 (95% CI: 0.57–0.96) p = 0.0217 | (ITT) ATEZO + BEV: 36.1 (31.5–42.3) SUN: 35.3 HR: 0.91 (95% CI: 0.76–1.08) p = 0.27 |
Checkmate 214 NCT02231749 Median follow-up of 60 months | 1096 | NIVO 3 mg/kg plus IPI 1 mg/kg IV Q3W for 4 doses then NIVO 3 mg/kg IV Q2W vs. SUN 50 mg | PFS and OS in the IMDC intermediate and poor risk population by BICR | IMDC FAV 23% IMD 61% POOR 17% MSKCC Not determined | (IMDC IMD/poor) NIVO + IPI: 11.6 (8.4–16.5) SUN: 8.3 (7.0–10.4) HR: 0.73 (95% CI: 0.61–0.87) | (IMDC IMD/poor) NIVO + IPI: 47.0 (35.4–57.4) SUN: 26.6 (22.1–33.5) HR: 0.68 (0.58–0.81) p < 0.0001 |
CheckMate 9ER NCT03141177 Median follow-up of 23.5 months | 651 | NIVO 240 mg fixed dose IV every 2 wk plus CABO 40 mg PO daily vs. SUN 50 mg | PFS in the ITT by BICR | IMDC FAV 22% IMD 58% POOR 20% MSKCC Not determined | (ITT) NIVO + CABO: 17.0 (12.6–19.4) SUN: 8.3 (6.9–9.7) HR: 0.52 (95% CI: 0.43–0.64) p < 0.0001 | (ITT) NIVO + CABO: SUN: 29.5 (28.4–NE) HR: 0.66 (98.9% CI: 0.50–0.87) p = 0.0034 |
CLEAR NCT02811861 Median | 712 | PEMBRO vs. SUN 50 mg | PFS in the ITT by BIRC | IMDC FAV 31% IMD 59% POOR 9% NE 1% MSKCC FAV 27% IMD 64% POOR 9% | (ITT) PEMBRO + LEN: 23.9 (20.8–27.7) SUN: 9.2 (6.0–11.0) HR: 0.39 (95% CI: 0.32–0.49) p > 0.001 | (ITT) PEMBRO + LEN: SUN: NR (38.4–E) HR: 0.72 (95% CI: 0.55–0.93) |
COSMIC-313 Median [521] | 855 | NIVO 3 mg/kg plus IPI 1 mg/kg vs. NIVO 3 mg/kg | PFS in the PITT population (first 550 pts. randomised) | IMDC IMD 75% POOR 25% | (PITT) NIVO + IPI + CABO: NR (14.0–NE) NIVO + IPI: HR: 0.73 (95% | NR |
ATEZO = atezolizumab; AVE = avelumab; AXI = axitinib; BEV = bevacizumab; BICR = blinded independentcentral review; BID = twice a day; CABO = cabozantinib; CI = confidence interval; FAV = favourable; HR = hazard ratio; IPI = ipilimumab; IMD = intermediate; IMDC = Metastatic Renal Cancer Database Consortium; IR = investigator review; ITT = intention-to-treat; IV = intravenous; LEN = lenvatinib; mo = months; MSKCC = Memorial Sloan Kettering Cancer Center; NE = non-estimable; NR = not reached; NIVO = nivolumab; OS = overall survival; PEMBRO = pembrolizumab; PFS = profession-free survival; PITT = PFS intention-to-treat; PO = by mouth; Pts = patients; QD = once a day; Q2W = every 2 weeks; Q3W = every 3 weeks; SUN = sunitinib; wk = weeks.
Patients who stop nivolumab plus ipilimumab because of toxicity require expert guidance and support from a multidisciplinary team before re-challenge can occur (LE: 1). Patients who do not receive the full four doses of ipilimumab due to toxicity should continue on single-agent nivolumab, where safe and feasible (LE: 4).
Treatment past progression with nivolumab plus ipilimumab can be justified but requires close scrutiny and the support of an expert multidisciplinary team [532,533] (LE: 1).
Patients who stop TKI and IO due to immune-related toxicity can receive single-agent TKI once the AE has resolved (LE: 1). Adverse event management, including transaminitis and diarrhoea, require particular attention as both agents may be causative. Expert advice should be sought on re-challenge of ICIs after significant toxicity (LE: 4). Treatment past progression on axitinib plus pembrolizumab or nivolumab plus cabozantinib requires careful consideration as it is biologically distinct from treatment past progression on ipilimumab and nivolumab.
Based on panel consensus, nivolumab plus ipilimumab, pembrolizumab plus axitinib and nivolumab plus cabozantinib and lenvatinib plus pembrolizumab should be administered in centres with experience of immune combination therapy and appropriate supportive care within the context of a multidisciplinary team (LE: 4).
7.4.4. Therapeutic strategies
7.4.4.1. Treatment-naïve patients with clear-cell metastatic RCC
The combination of pembrolizumab plus axitinib as well as nivolumab plus cabozantinib and lenvatinib plus pembrolizumab is the standard of care in all IMDC-risk patients and ipilimumab plus nivolumab in IMDC intermediate- and poor-risk patients (Figure 7.1). Therefore, the role of VEGFR-TKIs alone in front-line mRCC has been superseded. Sunitinib, pazopanib, and cabozantinib (IMDC intermediate- and poor-risk disease), remain alternative treatment options for patients who cannot receive or tolerate immune checkpoint inhibition in this setting (Figure 7.1).
7.4.4.1.1. Sequencing systemic therapy in clear-cell metastatic RCC
The sequencing of targeted therapies is established in mRCC and maximises outcomes [503,513]. Pembrolizumab plus axitinib, nivolumab plus cabozantinib, lenvatinib plus pembrolizumab and nivolumab plus ipilimumab are the new standard of care in front-line therapy. The impact of front-line immune checkpoint inhibition on subsequent therapies is unclear. Randomised data on patients with disease refractory to either nivolumab plus ipilimumab or TKI plus IO in a first-line setting are lacking, and available cohorts are limited [534]. Prospective data on tivozanib, cabozantinib and axitinib are available for patients progressing on immunotherapy, but these studies do not focus solely on the front-line setting, involve subset analyses, and are too small for definitive conclusions [513,535].
Retrospective data on VEGFR-TKI therapy after progression on front-line immune combinations exist but have significant limitations. When considering this data in totality, there is some activity but it is still too early to recommend one VEGFR-TKI over another after immunotherapy/immunotherapy or immunotherapy/VEGFR combination (Figure 7.2) [536,537]. After the axitinib plus pembrolizumab combination, changing the VEGFR-TKI at progression to cabozantinib or any other TKI not previously used is recommended.
The Panel do not support the use of mTOR inhibitors unless VEGF-targeted therapy is contra-indicated as they have been outperformed by other VEGF-targeted therapies in mRCC [538]. Drug choice in the third-line setting, after ICI combinations and subsequent VEGF-targeted therapy, is unknown. The Panel recommends a subsequent agent which is approved in VEGF-refractory disease, with the exception of re-challenge with immune checkpoint blockade. Cabozantinib is the only agent in VEGF-refractory disease with RCT data showing a survival advantage and should be used preferentially [500]. Axitinib has positive PFS data in VEGF-refractory disease. Both sorafenib and everolimus have been outperformed by other agents in VEGF-refractory disease and are therefore less attractive [538]. The lenvatinib plus everolimus combination appears superior to everolimus alone and has been granted EMA regulatory approval based on randomised phase II data. This is an alternative despite the availability of phase II data only [503]. As shown in a study which also included patients on ICIs, tivozanib provides PFS superiority over sorafenib in VEGF-refractory disease [539].
7.4.4.1.2. Summary of evidence and recommendations for immunotherapy in cc-mRCC
Summary of evidence | LE |
Treatment-naïve patients | |
Currently, PD-L1 expression is not used for patient selection. | 2b |
The combination of nivolumab and ipilimumab in treatment-naïve patients with cc-mRCC of IMDC intermediate- and poor risk demonstrated OS and ORR benefits compared to sunitinib alone. | 1b |
The combination of pembrolizumab plus axitinib, lenvatinib plus pembrolizumab and nivolumab plus cabozantinib in treatment-naïve patients with cc-mRCC across all IMDC risk groups demonstrated PFS, OS and ORR benefits compared to sunitinib. | 1b |
Nivolumab plus ipilimumab, pembrolizumab plus axitinib, nivolumab plus cabozantinib and lenvatinib plus pembrolizumab should be administered in centres with experience of immune combination therapy and appropriate supportive care within the context of a multidisciplinary team. | 4 |
The combination of nivolumab plus ipilimumab in the IMDC intermediate- and poor-risk population of treatment-naïve patients with cc-mRCC leads to superior survival compared to sunitinib. | 2b |
Sequencing systemic therapy | |
Nivolumab leads to superior OS compared to everolimus in disease progression after one or two lines of VEGF-targeted therapy. | 1b |
Axitinib, cabozantinib or lenvatinib can be continued if immune-related AEs result in cessation of axitinib plus pembrolizumab, cabozantinib plus nivolumab or lenvatinib plus pembrolizumab. Re-challenge with immunotherapy requires expert support. | 4 |
Patients who do not receive the full four doses of ipilimumab due to toxicity should continue on single-agent nivolumab, where safe and feasible. Re-challenge with combination therapy requires expert support. | 4 |
Treatment past progression can be justified but requires close scrutiny and the support of an expert multidisciplinary team. | 1b |
Nivolumab plus ipilimumab was associated with 46% grade 3–4 toxicity and 1.5% treatment-related deaths. Tyrosine kinase inhibitor-based IO combination therapies were associated with grade 3–5 toxicity ranging between 61–72% and 1% of treatment-related deaths. | 1b |
Recommendations | Strength rating |
Treatment-naïve patients | |
Offer treatment with PD1 combinations in centres with experience. | Weak |
Offer either nivolumab plus ipilimumab, pembrolizumab plus axitinib, or lenvatinib plus pembrolizumab, or nivolumab plus cabozantinib to treatment-naive patients with IMDC intermediate- or poor-risk disease. | Strong |
Offer either pembrolizumab plus axitinib, lenvatinib plus pembrolizumab or nivolumab plus cabozantinib to treatment-naïve patients with IMDC favourable risk. | Weak |
Offer sunitinib or pazopanib to treatment-naive patients with IMDC favourable risk. | Weak |
Offer sunitinib or pazopanib to treatment-naive cc-mRCC patients with any IMDC risk who cannot receive or tolerate immune checkpoint inhibition. | Strong |
Offer cabozantinib to treatment-naive patients with IMDC intermediate- and poor-risk cc-mRCC who cannot receive or tolerate immune checkpoint inhibition. | Stronga |
Patients who do not receive the full four doses of ipilimumab due to toxicity should continue on single-agent nivolumab, where safe and feasible. Re-challenge with combination therapy requires expert support. | Weak |
Sequencing systemic therapy | |
Sequence systemic therapy in treating mRCC. | Strong |
Offer VEGF-tyrosine kinase inhibitors as second-line therapy to patients refractory to nivolumab plus ipilimumab or axitinib plus pembrolizumab or cabozantinib plus nivolumab or lenvatinib plus pembrolizumab. | Weak |
Sequencing the agent not used as second-line therapy (nivolumab or cabozantinib) for third-line therapy is recommended. | Weak |
Offer nivolumab or cabozantinib to those patients who received first-line VEGF targeted therapy alone. | Strong |
Treatment past progression can be justified but requires close scrutiny and the support of an expert multi disciplinary team. | Weak |
Do not re-challenge patients who stopped immune checkpoint inhibitors because of toxicity without expert guidance and support from a multidisciplinary team. | Strong |
a While this is based on a randomised phase II trial, cabozantinib (weak) looks at least as good as sunitinib in this population. This justified the same recommendation under exceptional circumstances.
Figure 7.1: Updated EAU Guidelines recommendations for the first-line treatment of cc-mRCC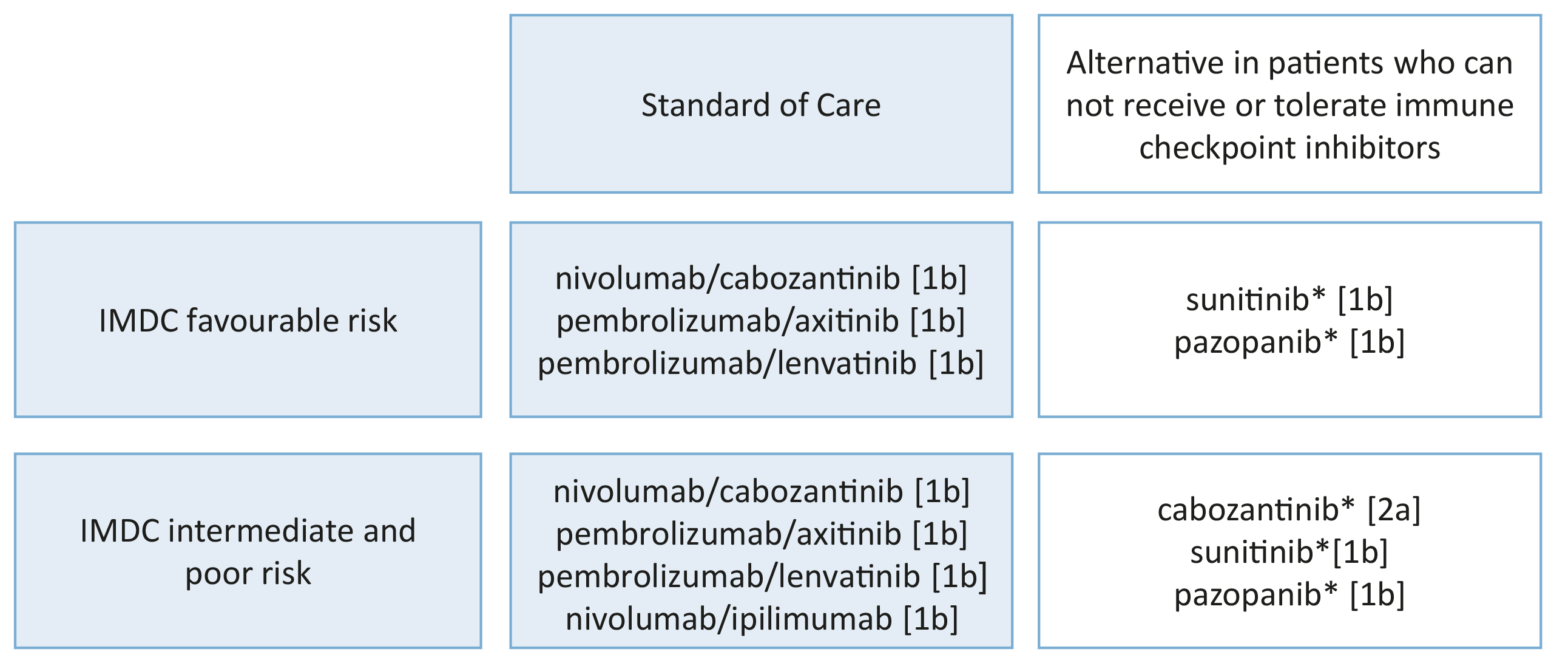 IMDC = The International Metastatic Renal Cell Carcinoma Database Consortium.
IMDC = The International Metastatic Renal Cell Carcinoma Database Consortium.
*pazopanib for intermediate-risk disease only.
[1b] = based on one randomised controlled phase III trial.
[2a] = based on a well-designed study without randomisation, or a subgroup analysis of a randomised controlled trial.
Figure 7.2: EAU Guidelines recommendations for later-line therapy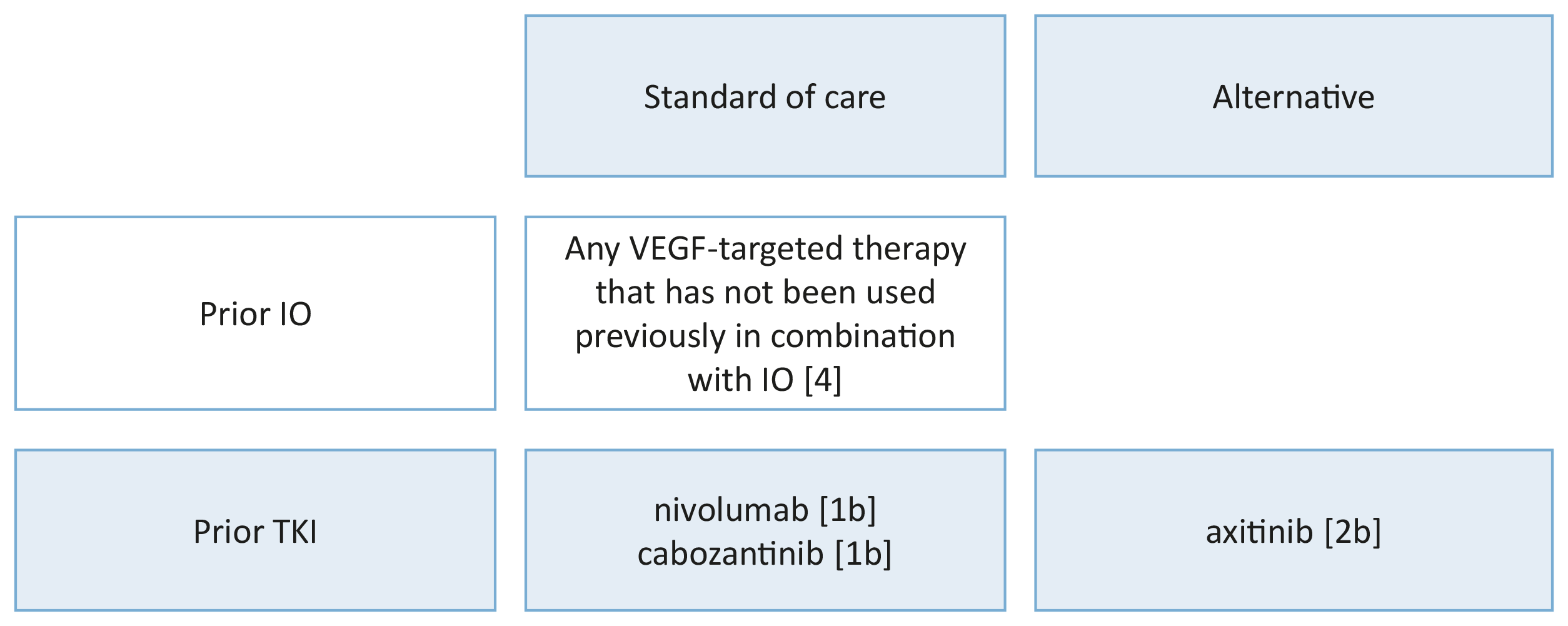 IO = immunotherapy; TKI = tyrosine kinase inhibitors; VEGF = vascular endothelial growth factor.
IO = immunotherapy; TKI = tyrosine kinase inhibitors; VEGF = vascular endothelial growth factor.
1b = based on one randomised controlled phase III trial.
2b = subgroup analysis of a randomised controlled phase III trial.
4 = expert opinion.
7.4.4.1.3. Renal tumours with sarcomatoid features
Subset analyses have shown improved results for PD-L1 inhibitors combined with CTLA4 or VEGF-targeted therapy in renal tumours with sarcomatoid features. Bevacizumab/atezolizumab, ipilimumab/nivolumab, axitinib/pembrolizumab and avelumab/axitinib can all be recommended instead of VEFG-targeted therapy alone. These options have OS advantages over sunitinib and superseded VEGF-targeted therapy.
Table 7.5: Subgroup analysis of first-line immune checkpoint inhibitor combinations in RCC patients with sarcomatoid histology
Cross trial comparison is not recommended and should occur with caution
Study | N (ITT) | Therapy | N (sRCC) | PFS (mo.) Median (95% CI) HR | OS (mo.) Median (95% CI) HR | ORR (%) (95% CI) |
KEYNOTE-426 NCT02853331 Median | 861 | PEMBRO + AXI SUN | 51 54 | NR 8.4 HR: 0.54 (0.29–1.00) | NR NR HR: 0.58 (0.21–1.59) | 58.8 31.5 |
JAVELIN 101 NCT02684006 | 886 | AVE + AXI SUN | 47 61 | 7.0 (5.3–13.8) 4.0 (2.7–5.7) HR 0.57 | NA | 46.8 (32.1–61.9) 21.3 (11.9–33.7) |
IMmotion151 NCT02420821 Median follow-up 13 to 17 mo. [543] | 915 | ATEZO + BEV SUN | 68 74 | 8.3 (5.4, 12.9) 5.3 (3.3, 6.7) HR: 0.52 | 21.7 (15.3, NE) 15.4 (10.4, 19.5) 0.64 (0.41, 1.01) | 49 (36–1) 14 (7–23) |
Checkmate 214 NCT02231749 Median follow-up of 30 mo. [544] | 1096 | NIVO + IPI SUN | 60 52 | 8.4 (5.2–24.0) 4.9 (4.0–7.0) HR: 0.61 (0.38–0.97) | 31.2 (23.0–NE) 13.6 (7.7–20.9) HR: 0.55 | 56.7 (43.2–69.4) 19.2 (9.6–32.5) |
CheckMate 9ER NCT03141177 Median follow-up | 651 | NIVO + CABO SUN | 34 41 | 10.3 (5.6–19.4) 4.2 (2.6–8.3) HR: 0.42 (0.23–0.74) | NR (22.8–NE) 19.7 (8.9–29.5) HR: 0.36 (0.17–0.79) | 55.9 (37.9–72.8) 22.0 (10.6–37.6) |
CLEAR NCT02811861 Median | 712 | PEMBRO + LEN SUN | 28 21 | 11.1 5.5 HR: 0.39 (0.18–0.84) | NE NE HR: 0.91 (0.32–2.58) | 60.7 23.8 |
ATEZO = atezolizumab; AVE = avelumab; AXI = axitinib; BEV = bevacizumab; CABO = cabozantinib;CI = confidence interval; HR = hazard ratio; IPI = ipilimumab; ITT = intention-to-treat; mo = months; NA = not available; NE = non-estimable; NR = not reached; NIVO = nivolumab; OS = overall survival; PEMBRO = pembrolizumab; PFS = profession-free survival; sRCC = sarcomatoid RCC; SUN = sunitinib.
7.4.4.1.3.1. Summary of evidence and recommendation for targeted therapy in RCC with sarcomatoid features
Summary of evidence | LE |
Immune checkpoint inhibitor combination therapy is superior to sunitinib in terms of PFS and OS in trial subset analysis of ccRCC with sarcomatoid features. | 2a |
Recommendation | Strength rating |
Offer immune checkpoint inhibitor combination therapy for advanced cc-mRCC with sarcomatoid features. | Weak |
7.4.4.2. Treatment of patients with non-clear-cell metastatic RCC
No phase III trials of patients with non-cc-mRCC have been reported. Expanded access programmes and subset analyses from RCC studies suggest the outcome of these patients with targeted therapy is poorer than for ccRCC. Treatment in non-cc-mRCC has focused on temsirolimus, everolimus, sorafenib, sunitinib, cabozantinib and pembrolizumab [547-550].
7.4.4.2.1. Summary of evidence and recommendation for targeted therapy in non-clear-cell metastatic RCC
Summary of evidence | LE |
Both mTOR inhibitors and VEGF-targeted therapies have limited activity in non-cc-mRCC. There is a non-significant trend for improved oncological outcomes for sunitinib over everolimus and for cabozantinib over sunitinib. | 2a |
In non-cc-mRCC, sunitinib improved PFS over everolimus in a systematic review of phase II trials and subgroups of patients. | 1a |
Recommendation | Strength rating |
Offer sunitinib to patients with other non-ccRCC subtypes than papillary RCC. | Weak |
7.4.4.3. Papillary metastatic RCC
The most common non-cc subtype is papillary RCC (pRCC). There are small single-arm trials for sunitinib and everolimus [550-554]. Both these agents have been widely given in pRCC, but more recent data suggests cabozantinib and other combinations may be preferable [555,556].
For pRCC new evidence is available from the SWOG PAPMET randomised phase II trial which compared sunitinib to cabozantinib, crizotinib and savolitinib in 152 patients with papillary mRCC [555]. Progression-free survival was longer in patients in the cabozantinib group (median 9.0 months, 95% CI: 6–12) than in the sunitinib group (5.6 months, CI: 3–7; HR for progression or death 0.60 [0.37–0.97, one-sided p = 0.019]). Response rate for cabozantinib was 23% vs. 4% for sunitinib (two-sided p = 0.010). Savolitinib and crizotinib did not improve PFS compared with sunitinib. Grade 3 or 4 AEs occurred in 69% (31/45) of patients receiving sunitinib, 74% (32/43) of patients receiving cabozantinib, 37% (10/27) receiving crizotinib, and 39% (11/28) receiving savolitinib; one grade 5 thromboembolic event was recorded in the cabozantinib group. These results support adding cabozantinib as an option for patients with papillary mRCC based on superior PFS results compared to sunitinib.
In addition, savolitinib was investigated in the SAVOIR trial [556] as first-line treatment for MET-driven tumours defined as chromosome 7 gain, MET amplification, MET kinase domain variations or hepatocyte growth factor amplification by DNA alteration analysis (~30% of screened patients were MET positive). In a limited patient group, savolitinib (n = 27) was compared with sunitinib (n = 33). The trial was stopped early, largely due to poor accrual. The efficacy data appeared to favour savolitinib (median PFS 7.0 months, 95% CI: 2.8 months–NR vs. 5.6 months, 95% CI: 4.1–6.9 months, PFS HR: 0.71, 95% CI: 0.37–1.36, OS HR: 0.51,94% CI: 0.21–1.17, RR: 27% vs. 7%, for savolitinib and sunitinib, respectively). The median OS for savolitinib was not reported, Savolitinib was better tolerated compared with sunitinib with 42% grade > 3 AEs compared to 81% with sunitinib. There are ongoing trials to confirm these findings. The results on these trials are required before recommendations can be made.
Early evidence for TKI + IO based combination is derived from two phase II studies of lenvatinib plus pembrolizumab and cabozantinib and nivolumab. The Keynote-B61 phase II trial investigated lenvatinib plus pembrolizumab administered to 51 patients with pRCC [557]. The primary endpoint of objective response was 52.9%, with a median follow-up of 8.2 months, providing some evidence of good efficacy for TKI + IO based combinations. The cabozantinib and nivolumab study enrolled 40 patients with papillary and unclassified RCC with a response rate of 48% and a PFS of 12.5 (6.3–15.9) months [558]. Indirect comparisons suggest these data compare favourably with those of VEGFR-TKI therapy alone.
Efficacy for pembrolizumab in the pRCC subset (118/165) was; RR: 29%, PFS: 5.5 months (95% CI: 3.9– 6.1 months) and OS: 31.5 months (95% CI: 25.5 months–NR), but these results are based on a single-arm phase II study [559]. Pembrolizumab can be considered in this setting due to the high unmet need; although the VEGFR TKI + IO combination may be preferable.
Patients with non-cc-mRCC should be referred to a clinical trial, where appropriate.
7.4.4.3.1. Summary of evidence and recommendation for targeted therapy in papillary metastatic RCC
Summary of evidence | LE |
Cabozantinib improved PFS over sunitinib in patients with advanced pRCC without additional molecular testing. | 2a |
Savolitinib improved PFS over sunitinib in patients with MET-driven advanced pRCC. | 2a |
Pembrolizumab resulted in long-term median OS in a single-arm study in the pRCC subgroup. | 2a |
Recommendations | Strength rating |
Offer cabozantinib to patients with papillary RCC (pRCC) based on a positive RCT. | Weak |
Offer pembrolizumab alone or lenvatinib plus pembrolizumab or nivolumab plus cabozantinib to patients with pRCC based on small single-arm trials. | Weak |
7.4.4.4. Treatment of patients with rare tumours
7.4.4.4.1. Renal medullary carcinoma
Renal medullary carcinoma is one of the most aggressive RCCs [29,194] and most patients (~67%) will present with metastatic disease [29,31]. Even patients who present with seemingly localised disease may develop macrometastases shortly thereafter, often within a few weeks.
Despite treatment, median OS is thirteen months in the most recent series [35]. Due to the infiltrative nature and medullary epicentre of RMC, RN is favoured over PN even in very early-stage disease. Retrospective data indicate that nephrectomy in localised disease results in superior OS (16.4 vs. 7 months) compared with systemic chemotherapy alone, but longer survival was noted in patients who achieved an objective response to first-line chemotherapy [35,560]. There is currently no established role for distant metastasectomy or nephrectomy in the presence of metastases.
Palliative radiation therapy is an option and may achieve regression in the targeted areas but it will not prevent progression outside the radiation field [561,562]. Renal medullary carcinoma is refractory to monotherapies with targeted anti-angiogenic regimens including TKIs and mTOR inhibitors [35,169]. The mainstay systemic treatments for RMC are cytotoxic combination regimens which produce partial or complete responses in ~29% of patients [169]. There are no prospective comparisons between different chemotherapy regimens, but most published series used various combinations of platinum agents, taxanes, gemcitabine, and/or anthracyclines [35,36]. High-dose-intensity combination of MVAC has also shown efficacy against RMC [563] although a retrospective comparison did not show superiority of MVAC over cisplatin, paclitaxel, and gemcitabine [36]. Single-agent anti-PD-1 immune checkpoint therapy has produced responses in a few case reports, although, as yet, insufficient data are available to determine the response rate to this approach [561,562]. Whenever possible, patients should be enrolled in clinical trials of novel therapeutic approaches, particularly after failing first-line cytotoxic chemotherapy.
7.4.4.5. Treatment of hereditary RCC
7.4.4.5.1.von-Hippel-Lindau-disease-associated RCC
Patients with VHL disease often develop RCC and tumours and cysts in other organs including adrenal glands, CNS, retinal haemangioblastomas, and pancreas, and commonly undergo several surgical resections in their lifetime. In VHL disease, belzutifan, a HIF-2α inhibitor, has been approved by the US Food and Drug Administration (FDA, August 2021) for the treatment of ccRCC and other neoplasms associated with VHL for the treatment of tumours that do not require immediate surgery. Approval was based on the results from a phase II, open-label, single-arm trial in 61 patients with tumours not larger than 3 cm [564]. Belzutifan induced partial responses with an RCC ORR of 49%, and a disease control rate of 98.4% after 21.8 months treatment. All patients with pancreatic lesions had an ORR of 77%, and those with CNS haemangioblastoma had a 30% response rate. In total, 33% of patients reported > grade 3 AEs, and seven patients (11.5%) discontinued the treatment. In the treatment with pazopanib for VHL only 52% continued with the treatment after 24 weeks [565]. A longer follow-up at 37.8 months, ORR for RCC was increased to 64%, with a median time to response of 11.1 months (range, 2.7 to 30.5). Median duration of response per Kaplan-Meier estimate was not reached (range, 5.4+ to 35.8+ months). Thirty-four of 39 patients with a confirmed response (87%) remain in response as of the data cut-off date (September 2022) [566].
With favourable efficacy results and with relatively low-grade side effects, belzutifan seems to be a valuable contribution to the treatment of patients with the VHL disease. The EMA has not yet considered belzutifan for approval in VHL disease, due to the limited safety data currently available.
7.5. Locally-recurrent RCC after treatment of localised disease
Most studies reporting on local recurrent disease after removal of the kidney have not considered the true definition of local recurrence after RN, PN and thermal ablation, which are: local recurrence in the tumour-bearing kidney, tumour growth exclusively confined to the true renal fossa, recurrences within the renal vein, the ipsilateral adrenal gland or the regional LNs. In the existing literature the topic is weakly investigated and often regarded as local recurrent disease.
7.5.1. Locally-recurrent RCC after nephron-sparing approaches
Locally-recurrent disease can affect the tumour-bearing kidney after PN or focal ablative therapy such as RFA and cryotherapy. Local relapse may be due to the incomplete resection of the primary tumour, in a minority of the cases to the local spread of the tumour by microvascular embolisation, or true multifocality [208,567].
The prognosis of recurrent disease not due to multifocality is poor, despite salvage nephrectomy [567]. Recurrent tumour growth in the regional LNs or ipsilateral adrenal gland may reflect metachronous metastatic spread (see Section 7.3). After treatment solely for localised disease, systemic progression is common [568,569].
Following thermal ablation or cryotherapy generally intra-renal, but also peri-renal, recurrences have been reported in up to 14% of cases [570]. Whereas repeat ablation is still recommended as the preferred therapeutic option after treatment failure, the most effective salvage procedure as an alternative to complete nephrectomy has not yet been defined.
7.5.2. Locally-recurrent RCC after radical nephrectomy
Isolated local fossa recurrence is rare and occurs in about 1–3% after radical nephrectomy. More commonly in pT3–4 than pT1–2 and grade 3–4 disease. Most patients with local recurrence of RCC are diagnosed by either CT/MRI scans as part of the post-operative follow-up [571]. The median time to recurrence after RN was 19–36 months in isolated local recurrence or 14.5 months in the group including metastatic cases as well [571-573].
Isolated local recurrence is associated with worse survival [208,574]. Based on retrospective and non-comparative data only, several approaches such as surgical excision, radiotherapy, systemic treatment and observation have been suggested for the treatment of isolated local recurrence [575-577]. Among these alternatives, surgical resection with negative margins remains the only therapeutic option shown to be associated with improved survival [574]. Open surgery has been successfully reported in studies [578,579]. One of the largest series including 2,945 patients treated with RN reported on 54 patients with recurrent disease localised in the renal fossa, the ipsilateral adrenal gland or the regional LNs as sole metastatic sites [575]. Another series identified 33 patients with isolated local recurrences and 30 local recurrences with synchronous metastases within a cohort of 2,502 surgically treated patients, confirming the efficacy of locally-directed treatment vs. conservative approaches (observation, systemic therapy) [580].
The 5-year OS with isolated local recurrence was 60% (95% CI: 0.44–0.73) and 10-year OS was 32% (95% CI: 0.15–0.51). Overall survival differed significantly by the time period between primary surgery and occurrence of recurrence (< 2 years vs. > 2 years: 10-year OS rate 31% (95% CI: 10.2–55.0) vs. 45% (95% CI: 21.5–65.8; HR: 0.26; p = 0.0034) [571]. Metastatic progression was observed in 60 patients (58.8%) after surgery [572]. Patient survival can be linked to the type of treatment received, as shown by Marchioni,
et al. [573]. In a cohort of 96 patients, 45.8% were metastatic at the time of recurrence; 3-year CSS rates after local recurrence were 92.3% ± 7.4%) for those who were treated with surgery and systemic therapy, 63.2%± 13.2%) for those who only underwent surgery, 22.7% ± 0.9%) for those who only received systemic therapy and 20.5% ± 10.4%) for those who received no treatment (p < 0.001).
However, minimally-invasive approaches, including standard and hand-assisted laparoscopic- and robotic approaches for the resection of isolated RCC recurrences have been occasionally reported. Recently, Martini et al., published the largest surgical cohort of robotic surgery in this setting (n = 35) providing a standardisation of the nomenclature, describing the surgical technique for each scenario and reporting on complications, renal function, and oncologic outcomes [581]. Ablative therapies including cryoablation, radiofrequency and microwave ablation, may also have a role in managing recurrent RCC patients, but further validation will be needed [582,583].
In summary, the limited available evidence suggests that in selected patients surgical removal of locally-recurrent disease with negative margins can induce durable tumour control, although with expected high risk of complications. Johnson et al., published on 51 planned repeat PNs in 47 patients with locally-recurrent disease, reporting a total of 40 peri-operative complications, with temporary urinary extravasation being the most prevalent [584]. Since local recurrences develop early, with a median time interval of 10–20 months after treatment of the primary tumour [585], a guideline-adapted follow-up scheme for early detection is recommended (see Chapter 8 - Follow-up) even though benefit in terms of cancer control has not yet been demonstrated [586].
Adverse prognostic parameters are a short time interval since treatment of the primary tumour (< 3–12 months) [587], sarcomatoid differentiation of the recurrent lesion and incomplete surgical resection [575]. In case complete surgical removal is unlikely to be performed or when significant comorbidities are present (especially when combined with poor prognostic tumour features), palliative therapeutic approaches including radiation therapy aimed at symptom control and prevention of local complications should be considered (see Sections 7.3 and 7.4). Following metastasectomy of local recurrence after nephrectomy, adjuvant therapy can be considered (see Section 7.2.5. Neoadjuvant and adjuvant therapy). Local recurrence combined with other metastases is treated as a metastatic RCC.
7.5.3. Summary of evidence and recommendation on locally-recurrent RCC after treatment of localised disease
Summary of evidence | LE |
Isolated recurrence after nephron-sparing procedures or nephrectomy is a rare entity (< 2%). | 3 |
Surgical or percutaneous treatment of local recurrences in absence of systemic progression should be considered, especially in absence of adverse prognostic parameters and favourable performance status. | 3 |
The most optimal modality of local treatment for locally-recurrent RCC after nephron-sparing procedures or nephrectomy is not defined. | 3 |
Recommendation | Strength rating |
Offer local treatment of locally-recurrent disease when technically possible and after balancing adverse prognostic features, comorbidities and life expectancy. | Weak |