5. DIAGNOSIS
5.1. Patient history
A focused patient history is mandatory.
5.2. Signs and symptoms
Haematuria is the most common finding in NMIBC. Visible haematuria was found to be associated with higher stage at diagnosis disease compared to nonvisible haematuria [90]. Carcinoma in situ might be suspected in patients with lower urinary tract symptoms, especially irritative voiding symptoms.
5.3. Physical examination
A focused urological examination is mandatory although it does not reveal NMIBC.
5.4. Imaging
5.4.1. Computed tomography urography and intravenous urography
Computed tomography (CT) urography is used to detect papillary tumours in the urinary tract, indicated by filling defects and/or hydronephrosis [91].
Intravenous urography (IVU) is an alternative if CT is not available [92] (LE: 2b), but particularly in muscle-invasive tumours of the bladder and in UTUCs, CT urography provides more information (including status of lymph nodes and neighbouring organs).
The necessity to perform a baseline CT urography once a bladder tumour has been detected is questionable due to the low incidence of significant findings which can be obtained [93-95] (LE: 2b). The incidence of UTUCs is low (1.8%), but increases to 7.5% in tumours located in the trigone [94] (LE: 2b). The risk of UTUC during follow-up increases in patients with multiple and high-risk tumours [96] (LE: 2b).
5.4.2. Ultrasound
Ultrasound (US) may be performed as an adjunct to physical examination as it has moderate sensitivity to a wide range of abnormalities in the upper- and lower urinary tract. It permits characterisation of renal masses, detection of hydronephrosis, and visualisation of intraluminal masses in the bladder, but cannot rule out all potential causes of haematuria [97,98] (LE: 3). It cannot reliably exclude the presence of UTUC and cannot replace CT urography.
5.4.3. Multi-parametric magnetic resonance imaging
The role of multi-parametric magnetic resonance imaging (mpMRI) has not yet been established in BC diagnosis and staging. A standardised methodology of MRI reporting (Vesical Imaging-Reporting and Data System [VI-RADS]) in patients with BC has recently been published and requires further validation [99]. A first systematic review of 8 studies showed that the VI-RADS scoring system can accurately differentiate NMIBC from MIBC with high inter-observer agreement rates [100].
A diagnosis of CIS cannot be made with imaging methods alone (CT urography, IVU, US or MRI) (LE: 4).
5.5. Urinary cytology
The examination of voided urine or bladder-washing specimens for exfoliated cancer cells has high sensitivity in HG and G3 tumours (84%), but low sensitivity in LG/G1 tumours (16%) [101]. The sensitivity in CIS detection is 28–100% [102] (LE: 1b). Cytology is useful, particularly as an adjunct to cystoscopy, in patients with HG/G3 tumours; it is not designed to detect LG tumours. Positive voided urinary cytology can indicate an UC anywhere in the urinary tract; negative cytology, however, does not exclude its presence.
Cytological interpretation is user-dependent [103,104] and evaluation can be hampered by low cellular yield, urinary tract infections, stones, or intravesical instillations: although in experienced hands specificity exceeds 90% [103] (LE: 2b). Artificial intelligence algorithms combined with digital image processing (VisioCyt test) improved the sensitivity of cytology for HG tumours up to 92% [105].
A standardised reporting system known as The Paris System published in 2022 (2nd Edn.) redefined urinary cytology diagnostic categories as follows [106]:
- No adequate diagnosis possible (No diagnosis);
- Negative for UC (Negative);
- Atypical urothelial cells (Atypia);
- Suspicious for HG UC (Suspicious);
- High-grade/G3 UC (Malignant).
The principle of the system and its terminology underlines the role of urinary cytology in detection of G3 and HG tumours. The Paris system for reporting urinary cytology has been validated in several retrospective studies [107,108].
Urine collection should respect the recommendation provided in Section 5.9. One cytospin slide from the sample is usually sufficient [109]. In patients with suspicious cytology repeat investigation is advised [110] (LE: 2b).
5.6. Urinary molecular marker tests
Driven by the low sensitivity of urine cytology in LG/G1 tumours, numerous urinary tests have been developed [111]. None of these markers have been accepted as routine practice by any clinical guidelines for diagnosis or follow-up.
The following conclusions can be drawn regarding the existing tests:
- Sensitivity is usually higher at the cost of lower specificity compared to urine cytology [112-117] (LE: 3).
- Benign conditions and previous BCG instillations may influence the results of many urinary marker tests [112-114] (LE: 1b).
- Requirements for sensitivity and specificity of a urinary marker test largely depend on the clinical context of the patient (screening, primary detection, follow-up [high-risk, low/intermediate-risk]) [113,114] (LE: 3).
- The wide range in performance of the markers and low reproducibility may be explained by patient selection and complicated laboratory methods required [114,115,118-125].
- Positive results of cytology, UroVysion™ (FISH), Nuclear Matrix Protein (NMP)22®, Fibroblast Growth Factor Receptor (FGFR)3/Telomerase Reverse Transcriptase (TERT) and microsatellite analysis in patients with negative cystoscopy and upper tract work-up, may identify patients more likely to experience disease recurrence and possibly progression [118,121,124-129] (LE: 2b).
- Promising novel urinary biomarkers, assessing multiple targets, have been tested in prospective multicentre studies [119,120,124,130-133]. Four of the promising and commercially available urine biomarkers, Cx-Bladder [119,133], ADX-BladderTM [134,135], Xpert Bladder® [136,137] and EpiCheckTM [132], although not tested in RCTs, have such high sensitivities and negative predictive values in the referenced studies for HG disease that these biomarkers may approach the sensitivity of cystoscopy. These 4 tests might be used to replace and/or postpone cystoscopy as they may identify the rare HG recurrences occurring in low/intermediate NMIBC.
5.7. Potential application of urinary cytology and markers
The following objectives of urinary cytology or molecular tests must be considered.
5.7.1. Screening of the population at risk of bladder cancer
The application of haematuria dipstick, followed by FGFR3, NMP22® or UroVysionTM tests if dipstick is positive has been reported in BC screening in high-risk populations [138,139]. However, the low incidence of BC in the general population and the short lead-time impair feasibility and cost-effectiveness of BC screening [127,139]. Thus, routine screening for BC is not recommended [127,138,139].
5.7.2.Exploration of patients after haematuria or other symptoms suggestive of bladder cancer (primary detection)
IIt is generally accepted that none of the currently available tests can replace cystoscopy. However, urinary cytology or biomarkers can be used as an adjunct to cystoscopy to detect missed tumours, particularly CIS. In this setting, specificity is particularly important. Recently, CellDetect® and UroVysion™ have shown similar performance to detect BC and were both superior to cytology [140]. In addition, Xpert Bladder® had higher sensitivity and negative-predictive value than both cytology or UroVysion™ for the detection of BC in patients with haematuria [141].
5.7.3. Surveillance of non-muscle-invasive bladder cancer
Research has been carried out into the usefulness of urinary cytology vs. markers in the follow-up of NMIBC [118,119,131,132,142]. A recent systematic review and network meta-analysis showed high accuracy of novel urinary biomarkers supporting their utility in the NMIBC surveillance setting [8].
5.7.3.1. Follow-up of high-risk non-muscle-invasive bladder cancer
High-risk tumours should be detected early in follow-up and the percentage of tumours missed should be as low as possible. Therefore, the best surveillance strategy for these patients will continue to include cystoscopy and cytology (see Chapter 8).
5.7.3.2. Follow-up of low/intermediate-risk non-muscle-invasive bladder cancer
To reduce the number of cystoscopy procedures, urinary markers should be able to detect a recurrence before the tumours are large, numerous and/or muscle invasive. The limitation of urinary cytology and current urinary markers is their low sensitivity for LG recurrences [113,118] (LE: 1b).
According to current knowledge, no urinary marker can replace cystoscopy during follow-up or lower cystoscopy frequency in a routine fashion. One prospective randomised study (RCT) found that knowledge of positive test results (microsatellite analysis) can improve the quality of follow-up cystoscopy [143] (LE: 1b), supporting the adjunctive role of a non-invasive urine test performed prior to follow-up cystoscopy [143] (see Section 8.1).
5.8. Cystoscopy
The diagnosis of papillary BC ultimately depends on cystoscopic examination of the bladder and histological evaluation of sampled tissue by either cold-cup biopsy or resection. Carcinoma in situ can be suspected through cystoscopy and urine cytology and confirmed by histological evaluation of multiple bladder biopsies [144].
Cystoscopy is initially performed as an outpatient procedure. A flexible instrument with topical intraurethral anaesthetic lubricant instillation results in better compliance compared to a rigid instrument, especially in men [145,146] (LE: 1b).
To temporary increase the urethral pressure by irrigation ‘bag squeeze’ when passing membranous and prostatic urethra with a flexible cystoscope in males also decreases pain during the procedure [147,148].
Figure 5.1: Bladder diagram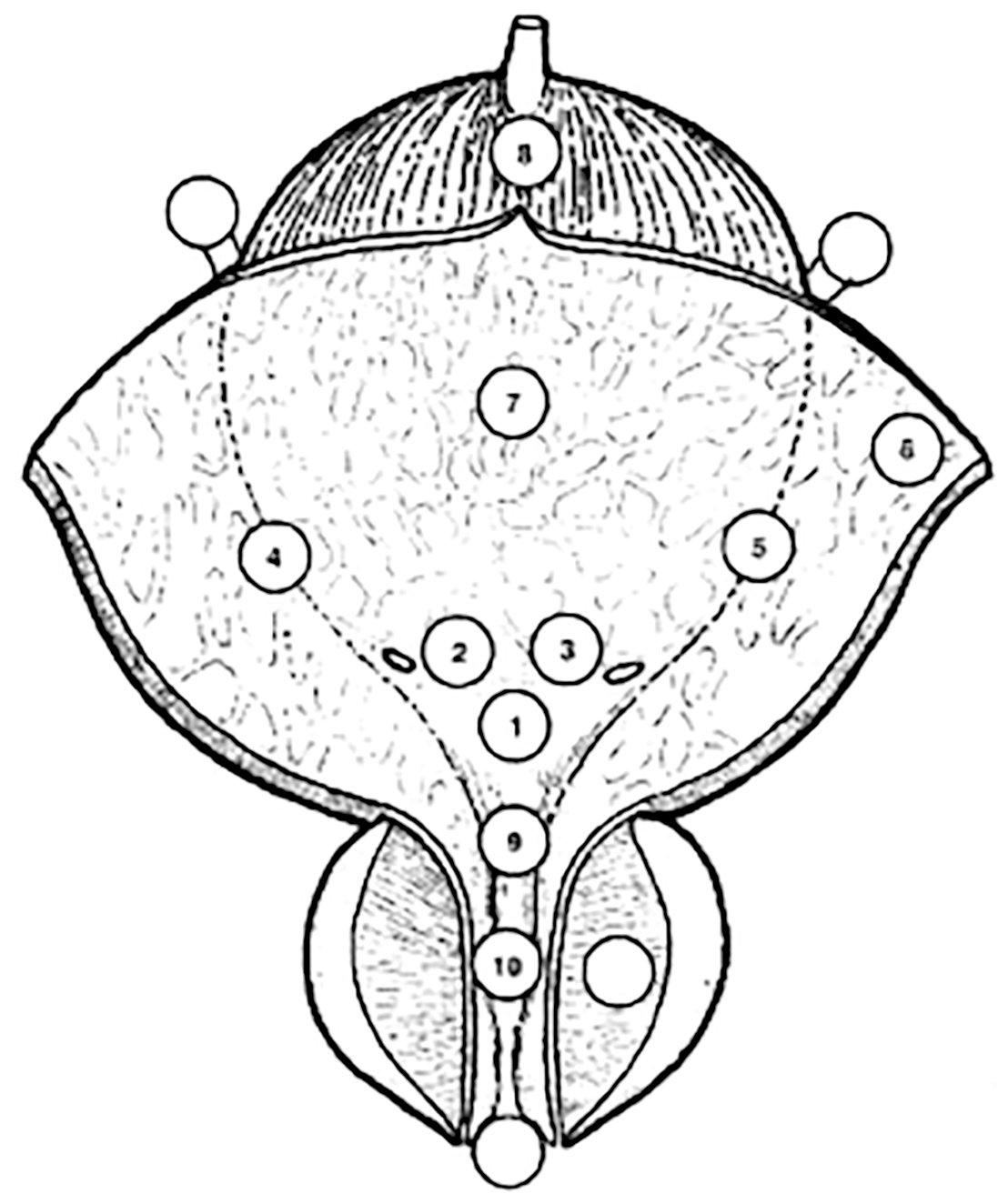
5.9. Summary of evidence and guidelines for the primary assessment of non-muscle-invasive bladder cancer
Summary of evidence | LE |
Cystoscopy is necessary for the diagnosis of BC. | 1 |
Urinary cytology has high sensitivity in high-grade tumours including carcinoma in situ. | 2b |
Recommendations | Strength rating |
Take a patient history, focusing on urinary tract symptoms and haematuria. | Strong |
Use renal and bladder ultrasound and/or computed tomography-intravenous urography (CT-IVU) during the initial work-up in patients with haematuria. | Strong |
Once a bladder tumour has been detected, perform a CT urography in selected cases (e.g., tumours located in the trigone, multiple- or high-risk tumours). | Strong |
Perform cystoscopy in patients with symptoms suggestive of bladder cancer or during surveillance. It cannot be replaced by cytology or by any other non-invasive test. | Strong |
In men, use a flexible cystoscope, if available, and apply irrigation ‘bag squeeze’ to decrease procedural pain when passing the proximal urethra. | Strong |
Describe all macroscopic features of the tumour (site, size, number, and appearance) and mucosal abnormalities during cystoscopy. Use a bladder diagram (Figure 5.1). | Strong |
Use voided urine cytology as an adjunct to cystoscopy to detect high-grade tumour. | Strong |
Perform cytology on at least 25 mL fresh urine or urine with adequate fixation. First morning urine is not suitable because of the frequent presence of cytolysis. | Strong |
Use the Paris System 2nd Edn. for cytology reporting. | Strong |
5.10. Transurethral resection of TaT1 bladder tumours
5.10.1. Strategy of the procedure
The goals of TURB in TaT1 BC is to establish accurate pathological diagnosis/staging and completely remove all visible lesions. It is a crucial procedure in the management of BC. Transurethral resection of the bladder tumours should be performed systematically in individual steps [6,149] (see Section 5.14).
The operative steps necessary to achieve a successful TURB include identifying the factors required to assign disease risk (number of tumours, size, architecture, location, concern for the presence of CIS, recurrent vs. primary tumour), clinical stage (bimanual examination under anaesthesia, assignment of clinical tumour stage), adequacy of the resection (visually complete resection, visualisation of muscle at the resection base), visualisation of tumour in the distal ureter and presence of complications (assessment for perforation) [6,150]. To measure the size of the largest tumour, one can use the end of the cutting loop, which is approximately 1 cm wide, as a reference. Tumour architecture can be sessile, nodular, papillary, mixed papillary/solid or flat.
5.10.2. Surgical and technical aspects of tumour resection
5.10.2.1. Surgical strategy of resection (piecemeal/separate resection, en-bloc resection)
A complete resection, performed by either fractioned or en-bloc technique, is essential to achieve a good prognosis [149,151].
- Piecemeal resection in fractions (separate resection of the exophytic part of the tumour, the underlying bladder wall and the edges of the resection area) provides good information about the vertical and horizontal extent of the tumour [152] (LE: 2b). Whilst this technique is carried out using a loop with diathermy (monopolar or bipolar), the Thulium-YAG laser is potentially a feasible alternative [153]. En-bloc resection using monopolar or bipolar current, Thulium-YAG or Holmium-YAG or KTP-Green Light lasers is feasible in selected exophytic tumours. It provides high-quality resected specimens with the presence of detrusor muscle in 96–100% of cases [149,154-160] (LE: 3). Respect of tumour architecture increases the accuracy of T1 staging and the possibility of sub-staging while potentially reducing the risk of bladder perforation [154,159,160] (LE: 1b).
The technique selected is dependent on the size and location of the tumour and experience of the surgeon. With better detection of tumours and abnormal margins, methods of optical enhancement are expected to improve complete resection (see Section 5.11). A recent RCT [161] did not confirm a higher rate of detrusor muscle retrieval in en-bloc- compared to piecemeal resection [162].
5.10.2.2. Evaluation of resection quality
The absence of detrusor muscle in the specimen is associated with a significantly higher risk of residual disease, early recurrence, and tumour under-staging [163] (LE: 1b). The presence of detrusor muscle in the specimen is considered as a surrogate criterion of the resection quality [163] and is required (except in Ta LG/G1 tumours). Surgical checklists and quality performance indicator programmes have shown to increase surgical quality (accurate documentation of factors required to assign risk and sample detrusor muscle) and decrease recurrence rates [6,150,164,165]. The Panel have included a sample TURBT checklist in Table 5.1 (modified and adapted from [5] and [6]).
It has been shown that surgical experience can improve TURB results, which supports the role of teaching programmes [163,166]. Virtual training on simulators is an emerging approach [167]. Its role in the teaching process still needs to be established [6]. Surgical experience and/or volume has been associated with risk of complications [168], recurrence [169] and survival [170] in retrospective studies (LE: 3).
5.10.2.3. Monopolar and bipolar resection
Compared to monopolar resection, bipolar resection has been introduced to reduce the risk of complications (e.g., bladder perforation due to obturator nerve stimulation) and to produce better specimens. Currently, the results remain controversial [171-173], with significant inherent limitations due to selection bias, heterogeneity of surgical approach or inability to qualify surgeon experience. A systematic review of 13 RCTs (2,379 patients) showed no benefit of bipolar vs. monopolar TURB for efficacy and safety [173] while one meta-analysis of RCTs (n = 2,099) suggests a lower fall in haemoglobin and shorter hospital stay with bipolar resections [171] and another systematic review of RCTs and observational studies (n = 19,927) suggests lesser thermal artifacts in the specimen [172].
5.10.2.4. Resection of small papillary bladder tumours at the time of transurethral resection of the prostate (TURP)
It is not uncommon to incidentally detect bladder tumours during TURP in men with benign prostatic hyperplasia. Provided these tumours are papillary, rather small and not extensively multifocal, it seems feasible to resect these tumours and continue with the resection of the prostate [174,175]. Simultaneous TURB and TURP does not appear to lead to any increased risk of tumour recurrence or progression [176] (LE: 3).
5.11. Endoscopic biopsies
5.11.1. Bladder biopsies
Carcinoma in situ can present as a velvet-like, reddish area, indistinguishable from inflammation, or it may not be visible at all. For this reason, biopsies from suspicious urothelium should be taken. In patients with positive urine cytology (see Section 5.5), and normal-looking mucosa at cystoscopy, mapping biopsies are recommended [177,178]. To obtain representative mapping of the bladder mucosa, biopsies should be taken from the trigone, bladder dome, right, left, anterior and posterior bladder wall [177,178]. If the equipment is available, photodynamic diagnosis (PDD) is a useful tool to target the biopsy.
5.11.2. Prostatic urethral biopsies
Involvement of the prostatic urethra and ducts in men with NMIBC has been reported. Palou et al., showed that in 128 men with T1G3 UC, the incidence of CIS in the prostatic urethra was 11.7% [179] (LE: 2b). The risk of prostatic urethra or duct involvement is higher if the tumour is located at the trigone or bladder neck, in the presence of bladder CIS and multiple tumours [180] (LE: 3b). Based on this observation, a biopsy from the prostatic urethra is necessary in some cases (see recommendation in Section 5.14) [179,181,182].
5.12. New methods of tumour visualisation
As a standard procedure, cystoscopy and TURB are performed using white light (WL). However, the use of WL alone can lead to missing lesions that are present but not visible, which is why new technologies are being developed.
5.12.1. Photodynamic diagnosis (fluorescence cystoscopy or blue light cystoscopy)
Photodynamic diagnosis is performed using violet light after intravesical instillation of 5-aminolaevulinic acid (ALA) or hexaminolaevulinic acid (HAL).
5.12.1.1. Impact on bladder cancer detection
It has been confirmed that fluorescence-guided biopsy and resection are more sensitive than conventional procedures for the detection of malignant tumours, particularly CIS [183,184] (LE: 1a). In a systematic review and meta-analysis, PDD had higher sensitivity than WL endoscopy in the pooled estimates for analyses at both the patient-level (92% vs. 71%) and biopsy-level (93% vs. 65%) [184]. A prospective RCT did not confirm a higher detection rate in patients with known positive cytology before TURB [185].
Photodynamic diagnosis had lower specificity than WL endoscopy (63% vs. 81%) [184]. False-positivity can be induced by inflammation or recent TURB and during the first 3 months after BCG instillation [186,187] (LE: 1a).
5.12.1.2. Impact on bladder cancer recurrence
The beneficial effect of ALA or HAL fluorescence cystoscopy on recurrence rate in patients with TURB was evaluated. A systematic review and analysis of 14 RCTs including 2,906 patients, 6 using 5-ALA and 9 HAL, demonstrated a decreased risk of BC recurrence in the short and long term. There were, however, no differences in progression and mortality rates. The analysis demonstrated inconsistency between trials and potential susceptibility to performance and publication bias [188] (LE: 1a). While a recent systematic review and meta-analysis of 12 RCTs (n = 2,288) revealed lower risk of recurrence and improved time to recurrence (at least in the first 2 years and possibly up to 5 years) with PDD [189] (LE: 1a), the most recent Cochrane systematic review and meta-analysis of 16 RCTs (n = 4,325) demonstrated that PDD-assisted TURBT may prolong not only recurrence over time but also risk of progression, albeit supported only by low certainty evidence [190].
A recent RCT showed that PDD-guided TURBT did not reduce recurrence rates, nor was it cost-effective compared with WL cystoscopy at 3 years [191]. The study was considered underpowered, potentially having captured a diluted effect of PDD at 3 years (as opposed to the positive trend in time to recurrence seen within the first 18 months) [192-194].
5.12.2. Narrow-band imaging
In narrow-band imaging (NBI), the contrast between normal urothelium and hyper-vascular cancer tissue is enhanced. Improved cancer detection has been demonstrated by NBI flexible cystoscopy and NBI-guided biopsies and resection [195-198] (LE: 3b). Two RCTs assessed the reduction of recurrence rates if NBI is used during TURB [198,199]. Although the overall results were negative, a benefit after 3 and 12 months was observed for low-risk tumours (pTa LG, < 30 mm, no CIS) [199].
A systematic review and meta-analysis by Russo et al., (17 RCTs and non-RCTs) demonstrated improved detection (diagnostic accuracy) of bladder tumours with either PDD or NBI over WL cystoscopy [200], while another one (including 5,217 patients) showed improved RFS with either enhancement technique [201] (LE: 1a). Conversely, a systematic review and network meta-analysis that took into account the use of single post-operative instillation of chemotherapy, concluded that there was a lower likelihood of recurrence at one year only following PDD-guided TURB (with or without single instillation) but not with NBI-guided surgery [202] (LE: 1a).
5.12.3. IMAGE1 S™, and other technologies
IMAGE1 S™ (formerly named SPIES) is an image enhancement system based on a computerized processing of different colour components that uses specific light filters. Limited evidence has been produced so far in an attempt to validate the 4 different light spectra modalities, suggesting an improvement in the diagnostic accuracy of WL [203,204]. Early (18 months) follow-up data of an RCT failed to show an advantage in recurrence rate in the IMAGE1 S™ arm over WL, except in a subgroup of primary low intermediate-risk NMIBCs [205].
Confocal laser micro-endoscopy is a high-resolution imaging probe designed to provide endoscopic histological grading in real time but requires further validation [206].
5.13. Second resection (second TURB)
5.13.1. Detection of residual disease and tumour upstaging
The significant risk of residual tumour after initial TURB of TaT1 lesions has been demonstrated [151] (LE: 1b). This residual cancer has the potential to worsen oncological outcomes and therefore further emphasises the importance of an effective initial TURB. As patients with an initial incomplete TURB (either from extensive tumour or intra-operative complications) will require a second completion resection, documentation of resection completeness at the time of the initial TURB is essential.
A systematic review analysing data of 8,409 patients with Ta or T1 HG UC demonstrated a 51% risk of persistence and an 8% risk of under-staging in T1 tumours. The analysis also showed a high risk of residual disease in Ta tumours, but this observation was based only on a limited number of cases. Most of the residual lesions were detected at the original tumour location [207] (LE: 1a).
Another systematic review and meta-analysis of 3,556 patients with T1 tumours showed that the prevalence rate of residual tumours and upstaging to invasive disease after TURB remained high even in a subgroup with detrusor muscle sampled at the initial TURB. In the subgroup of 1,565 T1 tumours with detrusor muscle present, persistent tumour was found in 58% and under-staging occurred in 11% of cases [208].
Prospective trials suggest that post-operative positive urine cytology [209] and Xpert Bladder® (urine mRNA test) [210] are independently associated with residual disease at second resection and risk of future recurrences, respectively (LE: 2b). These data, however, need to be confirmed in further studies.
5.13.2. The impact of second resection on treatment outcomes
A second TURB can increase recurrence-free survival (RFS) [211,212] (LE: 2a), improve outcomes after BCG treatment [213] (LE: 3) and provide prognostic information [214-217] (LE: 3).
In a retrospective evaluation of a large multi-institutional cohort of 2,451 patients with BCG-treated T1 G3/HG tumours (a second resection was performed in 935 patients), the second resection improved RFS, progression-free survival (PFS) and overall survival (OS) only in patients without detrusor muscle in the initial resection specimen [218] (LE: 3). In a retrospective analysis of 7,666 patients diagnosed with T1 cancer in Ontario, 2,162 underwent a second resection; after adjusting for the effects of confounding variables, only OS (and not CSS) was better in patients who underwent second resection [170] (LE: 3). This apparent improved survival could also be the result of selection bias with fitter patients undergoing second resections. High-level evidence is required to identify specific sub-groups of patients with high-grade cancer who are most likely to benefit from a second resection.
5.13.3. Timing of second resection
Retrospective evaluation showed that a second resection performed 14–42 days after initial resection provides longer RFS and PFS compared to second resection performed after 43–90 days [219] (LE: 3). Based on this currently available evidence, a second TURB is recommended in selected cases 2 to 6 weeks after initial resection [219] (for recommendations on patient selection, see Section 5.14).
5.13.4. Recording of results
The results of the second resection (residual tumours and under-staging) reflect the quality and effectiveness of the initial TURB. As the goal is to improve the quality of the initial TURB, the results of the second resection should be recorded.
5.14. Pathology report
Pathological investigation of the specimen(s) obtained by TURB and biopsies is an essential step in the decision-making process for BC [220]. Close co-operation between urologists and pathologists is required. Clinical information and high quality of resected and submitted tissue is essential for correct pathological assessment. To obtain all relevant information, the specimen collection, handling and evaluation, should respect the recommendations provided below (see Section 5.14) [221]. In difficult cases, an additional review by an experienced genitourinary pathologist can be considered.
Table 5.1 TURBT checklist*
TURBT checklist - In the Operating Room | |
Check the operating room setup | Instruments (sheath, resectoscope, loops, roller if needed, monopolar/bipolar), camera, video, strainer, specimen container, catheter if needed |
Decide irrigation fluid | Saline, Glycine, Water |
Disease characteristics checklist | History of bladder cancer, tumour characteristics at cystoscopy if any, imaging results if any, first or second look, visual optimisation planned (PDD/NBI), risk classification |
Cystoscopy/ TURBT | |
Cystoscopy | Urethra/prostate (males) |
Ureteral orifices | |
Diverticuli | |
Tumour location, number, size, appearance (papillary/sessile), CIS (yes/no) | |
White light/PDD/NBI/IMAGE1 S™ | |
Urine for cytology/bladder wash | |
TURBT | Resection technique (standard/en bloc/cold cup/roller ball cautery) |
Depth of resection | |
Complete/incomplete resection | |
Prostatic urethra biopsy if performed | |
Any additional procedure, i.e. retrograde contrast study | |
Estimated blood loss | |
Intra-operative complications, if any | |
Intravesical therapy if given or planned in recovery setting | |
*Adapted from Mostafid et al., and Suarez-Ibarrola et al., [5, 6].
NBI = narrow-band imaging; PDD = photodynamic diagnosis; TURBT = transurethral resection of bladder tumour.
5.15. Summary of evidence and guidelines for transurethral resection of the bladder, biopsies and pathology report
Summary of evidence | LE |
Transurethral resection of the bladder tumour (TURB) followed by pathology investigation of the obtained specimen(s) is an essential step in the management of NMIBC. | 1 |
The absence of detrusor muscle in the specimen is associated with a significantly higher risk of residual disease and tumour under-staging (with the exception of Ta LG/G1 tumours). | 2b |
A second TURB can detect residual tumours and tumour under-staging, increase recurrence-free survival, improve outcomes after BCG treatment and provide prognostic information. | 2 |
Recommendations | Strength rating |
In patients suspected of having bladder cancer, perform a transurethral resection of the bladder tumour (TURB) followed by pathology investigation of the obtained specimen(s) as a diagnostic procedure and initial treatment step. | Strong |
Perform TURB systematically in individual steps: bimanual palpation under anaesthesia; insertion of the resectoscope, under visual control with inspection of the whole urethra; inspection of the whole urothelial lining of the bladder; biopsy from the prostatic urethra (if indicated); cold-cup bladder biopsies (if indicated); resection of the tumour; recording of findings in the surgery report/record; precise description of the specimen(s) for pathology evaluation. | Strong |
Performance of individual steps | |
Perform en-bloc resection or resection in fractions (exophytic part of the tumour, the underlying bladder wall and the edges of the resection area). | Strong |
Avoid cauterisation as much as possible during TURB to avoid tissue deterioration. | Strong |
Take biopsies from abnormal-looking urothelium. Biopsies from normal-looking mucosa (mapping biopsies from the trigone, bladder dome, right, left, anterior and posterior bladder wall) are recommended if cytology or urinary molecular marker test is positive. If the equipment is available, perform fluorescence-guided (PDD) biopsies. | Strong |
Take a biopsy of the prostatic urethra in cases of bladder neck tumour, if there is positive cytology or urinary molecular marker test without evidence of tumour in the bladder, or if abnormalities of the prostatic urethra are visible. If biopsy is not performed during the initial procedure, it should be performed at the time of the second resection. | Strong |
Take a prostatic urethral biopsy from the pre-collicular area (between the 5 and 7 o’clock position) using a resection loop. | Weak |
Use methods to improve tumour visualisation (fluorescence cystoscopy, narrow-band imaging) during TURB, if available. | Weak |
Refer the specimens from different biopsies and resection fractions to the pathologist in separately labelled containers. | Weak |
The TURB record must describe tumour location, appearance, size and multifocality, all steps of the procedure, extent, macroscopic completeness of resection as well as any complications. | Strong |
In patients with positive cytology, but negative cystoscopy, exclude an upper tract urothelial carcinoma, CIS in the bladder (by mapping biopsies or PDD-guided biopsies) and tumour in the prostatic urethra (by prostatic urethra biopsy). | Strong |
Perform a second TURB in the following situations: after incomplete initial TURB, or in case of doubt about completeness of a TURB; if there is no detrusor muscle in the specimen after initial resection, with the exception of Ta LG/G1 tumours and primary CIS; in T1 tumours. | Strong |
If indicated, perform a second TURB within 2–6 weeks after the initial resection. This second TURB should include resection of the primary tumour site. | Weak |
Register the pathology results of a second TURB as it reflects the quality of the initial resection. | Weak |
Inform the pathologist of prior treatments (intravesical therapy, radiotherapy, etc.). | Strong |
The pathological report should specify tumour location, tumour grade and stage, lympho-vascular invasion, subtypes of urothelial carcinoma, presence of CIS and detrusor muscle. | Strong |