1. INTRODUCTION
1.1. Aim and scope
This overview represents the updated European Association of Urology (EAU) Guidelines for Non-muscle-invasive Bladder Cancer (NMIBC), TaT1 and carcinoma in situ (CIS). The information presented is limited to urothelial carcinoma (UC), unless specified otherwise. The aim is to provide practical recommendations on the clinical management of NMIBC with a focus on clinical presentation and recommendations. Separate EAU Guidelines are available addressing upper tract urothelial carcinoma (UTUC) [1], muscle-invasive and metastatic bladder cancer (MIBC) [2] and primary urethral carcinoma [3]. It must be emphasised that clinical guidelines present the best evidence available to the experts, but following guideline recommendations will not necessarily result in the best outcome. Guidelines can never replace clinical expertise when making treatment decisions for individual patients, but rather help to focus decisions - also taking personal values and references/individual circumstances of patients into account. Guidelines are not mandates and do not purport to be a legal standard of care.
1.2. Panel composition
The EAU Guidelines Panel on NMIBC consists of an international multidisciplinary group of clinicians, including urologists, uro-oncologists, a pathologist, and a statistician. Members of this Panel have been selected based on their expertise and to represent the professionals treating patients suspected of suffering from bladder cancer. In the course of 2021 two patient representatives have formally joined the NMIBC Panel. All experts involved in the production of this document have submitted potential conflict of interest statements which can be viewed on the EAU website Uroweb: https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/.
1.3. Available publications
A quick reference document (Pocket guidelines) is available. This is an abridged version which may require consultation together with the full text version. Several scientific publications are available, the latest publication dating to 2022 [4], as are a number of translations of all versions of the EAU NMIBC Guidelines. All documents are accessible through the EAU website Uroweb: https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/.
1.4. Publication history and summary of changes
1.4.1. Publication history
The EAU Guidelines on Bladder Cancer were first published in 2000. This 2023 NMIBC Guidelines document presents a limited update of the 2022 publication.
1.4.2. Summary of changes
Additional data has been included throughout this document text. In particular in Chapters/Sections:
• Section 3.2 Aetiology, was restructured.
• Chapter 4 Pathological staging and classification systems, was significantly updated.
• The inclusion of the new WHO 2022 classification resulted in new Figure 4.1 Stratification of tumours according to grade in the WHO 1973 and 2004/2022 classifications;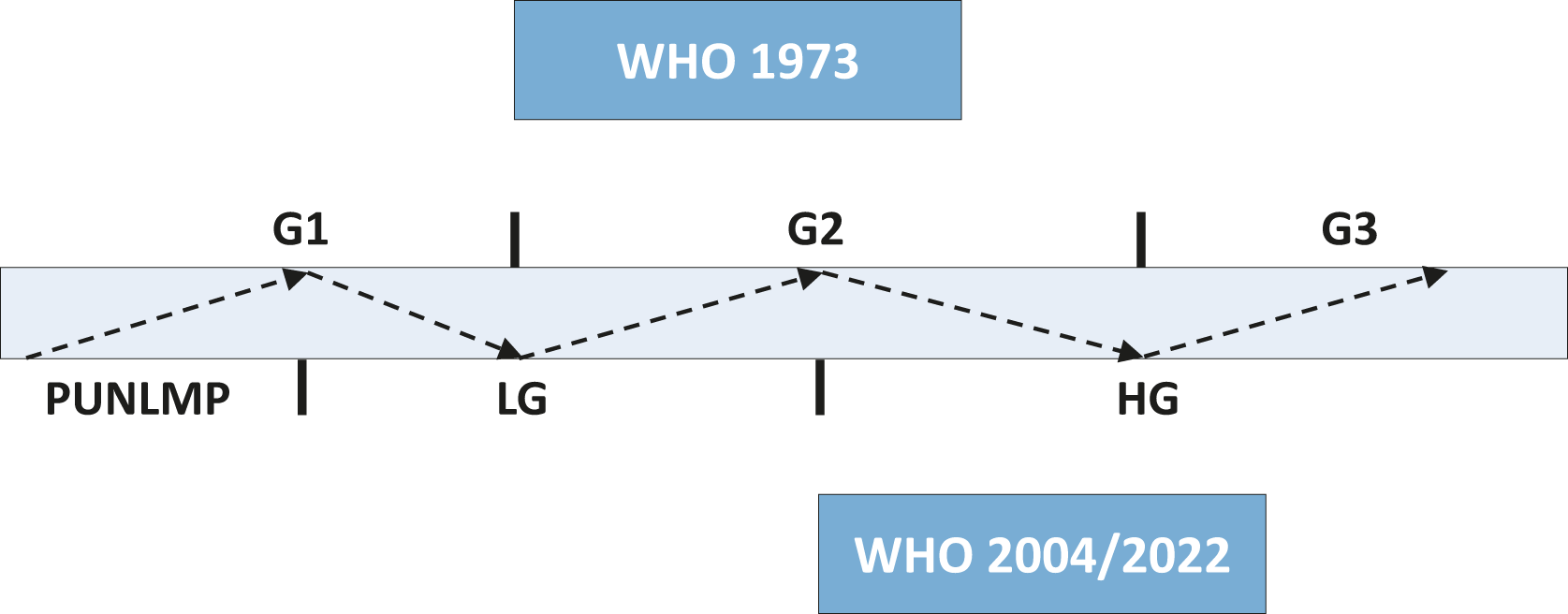
• Revision of Section 4.7 Subtypes of urothelial carcinoma.
• In Section 4.10 the following recommendation was revised:
Recommendations | Strength rating |
2022 recommendation: Use both the 1973 and 2004/2022 WHO classification systems. | Strong |
Revised 2023 recommendation: Use both the 1973 and 2004/2022 WHO classification systems, or a hybrid system. | Weak |
• Section 5.1 Transurethral resection of TaT1 tumours has been revised and restructured, in particular ‘New methods of tumour visualisation’, the inclusion of Table 5.1 TURBT checklist, with changes to recommendations:
Recommendations | Strength rating |
2022 recommendation: Take a biopsy of the prostatic urethra in cases of bladder neck tumour, if bladder carcinoma in situ is present or suspected, if there is positive cytology or urinary molecular marker test without evidence of tumour in the bladder, or if abnormalities of the prostatic urethra are visible. If biopsy is not performed during the initial procedure, it should be completed at the time of the second resection. | Strong |
Revised 2023 recommendation: Take a biopsy of the prostatic urethra in cases of bladder neck tumour, if there is positive cytology or urinary molecular marker test without evidence of tumour in the bladder, or if abnormalities of the prostatic urethra are visible. If biopsy is not performed during the initial procedure, it should be performed at the time of the second resection. | Strong |
2022 recommendation: Take a prostatic urethral biopsy from the pre-collicular area (between the 5 and 7 o’clock position) using a resection loop. In case any abnormal-looking areas in the prostatic urethra are present at this time, these need to be biopsied as well. | Weak |
Revised 2023 recommendation: Take a prostatic urethral biopsy from the pre-collicular area (between the 5 and 7 o’clock position) using a resection loop. | Weak |
2022 recommendation: The TURB record must describe tumour location, appearance, size and multifocality, all steps of the procedure, as well as extent and completeness of resection. | Weak |
Revised 2023 recommendation: The TURB record must describe tumour location, appearance, size and multifocality, all steps of the procedure, extent, macroscopic completeness of resection as well as any complications. | Strong |
Table 5.1 TURBT checklist*
TURBT checklist - In the Operating Room | |
Check the operating room setup | Instruments (sheath, resectoscope, loops, roller if needed, monopolar/bipolar), camera, video, strainer, specimen container, catheter if needed |
Decide irrigation fluid | Saline, Glycine, Water |
Disease characteristics checklist | History of bladder cancer, tumour characteristics at cystoscopy if any, imaging results if any, first or second look, visual optimisation planned (PDD/NBI), risk classification |
Cystoscopy/ TURBT | |
Cystoscopy | Urethra/prostate (males) |
Ureteral orifices | |
Diverticuli | |
Tumour location, number, size, appearance (papillary/sessile), CIS (yes/no) | |
White light/PDD/NBI/IMAGE1 S™ | |
Urine for cytology/bladder wash | |
TURBT | Resection technique (standard/en bloc/cold cup/roller ball cautery) |
Depth of resection | |
Complete/incomplete resection | |
Prostatic urethra biopsy if performed | |
Any additional procedure, i.e. retrograde contrast study | |
Estimated blood loss | |
Intra-operative complications, if any | |
Intravesical therapy if given or planned in recovery setting | |
*Adapted from Mostafid et al., and Suarez-Ibarrola et al. [5,6].
• Chapter 7 Disease management; new Sections 7.2 Office-based fulguration and laser vaporisation; 7.3 Active Surveillance; 7.4.1 Post-operative irrigation; Conductive chemo-hyperthermia; 7.4.4.3 Sequential chemotherapy instillations; in Section 7.7 Primary treatment by disease type, Treatment of carcinoma in situ was added; and a new Section 7.8 Multidisciplinary tumour board, resulting in changes to recommendations:
Recommendations | Strength rating |
2022 recommendation: Counsel smokers with confirmed non-muscle-invasive bladder cancer (NMIBC) to stop smoking. | Strong |
Revised 2023 recommendation: Counsel smokers to stop smoking. | Strong |
2022 recommendation: In patients with tumours presumed to be at low risk and in those with small papillary recurrences (presumably Ta LG/G1) detected more than one year after previous TURB, offer one immediate chemotherapy instillation. | Strong |
Revised 2023 recommendation: In patients with tumours presumed to be at low risk and in those with small papillary recurrences (presumably Ta LG/G1) detected more than one year after previous TURB, offer one immediate single chemotherapy instillation. | Strong |
New 2023 recommendation: Offer post-operative saline or water continuous irrigation of the bladder to patients who cannot receive a single instillation of chemotherapy. | Strong |
New 2023 recommendation: Patients with small recurrent low grade Ta tumours can be effectively and safely offered office fulguration. | Strong |
New 2023 recommendation: Only offer active surveillance to selected patients with presumed low-risk tumours not amendable to endoscopic ablation. | Strong |
2022 recommendation: In patients with intermediate-risk tumours (with or without immediate instillation), offer one-year full- dose Bacillus Calmette- Guérin (BCG) treatment (induction plus 3-weekly instillations at 3, 6 and 12 months), or instillations of chemotherapy (the optimal schedule is not known) for a maximum of one year. The final choice should be made in a shared decision-making process with the patient, reflecting his/her risk of recurrence and progression, as well as the efficacy and side effects of each treatment modality. | Strong |
Revised 2023 recommendation: In patients with intermediate-risk tumours (with or without immediate instillation), one-year full- dose Bacillus Calmette- Guérin (BCG) treatment (induction plus 3-weekly instillations at 3, 6 and 12 months), or instillations of chemotherapy (the optimal schedule is not known) for a maximum of one year is recommended. The final choice should reflect the individual patient’s risk of recurrence and progression as well as the efficacy and side effects of each treatment modality, in a shared decision-making process with the patient. | Strong |
2022 recommendation: In patients with high-risk tumours, full-dose intravesical BCG for one to 3 years (induction plus 3-weekly instillations at 3, 6, 12, 18, 24, 30 and 36 months), is indicated. The additional beneficial effect of the second and third years of maintenance should be weighed against its added costs, side effects and problems connected with BCG shortages. Immediate radical cystectomy (RC) may also be discussed with the patient. | Strong |
Revised 2023 recommendation: In patients with high-risk tumours, full-dose intravesical BCG for one to 3 years (induction plus 3-weekly instillations at 3, 6, 12, 18, 24, 30 and 36 months), is indicated. The additional beneficial effect of the second and third years of maintenance should be weighed against its added costs, side effects and access to BCG. Immediate radical cystectomy (RC) may also be discussed with the patient. | Strong |
2022 recommendation: In patients with very high-risk tumours discuss immediate RC. Offer intravesical full-dose BCG instillations for one to 3 years to those who refuse or are unfit for RC. | Strong |
Revised 2023 recommendation: In patients with very high-risk tumours offer immediate RC. Discuss intravesical full-dose BCG instillations for one to 3 years and discuss clinical trials with those who refuse or are unfit for RC. | Strong |
New 2023 recommendation: Cautiously offer quinolones to treat BCG-related side effects*. | Weak |
Recommendations - technical aspects for treatment | |
BCG intravesical immunotherapy | |
New 2023 recommendation: Discuss high-risk and very high-risk patients within a multidisciplinary board, when possible. | Weak |
*The side-effect profile of quinolones and fluoroquinolones resulted in the adoption of European regulation restricting their use [7].
• Chapter 8 Follow-up of patients with NMIBC, significant marker data in the follow-up setting has been included, as well as a new table 8.1 Urinary markers in the surveillance setting, resulting in the deletion of one recommendation:
Recommendation | Strength rating |
In patients initially diagnosed with Ta LG/G1–2 bladder cancer, use ultrasound of the bladder during surveillance in case cystoscopy is not possible or refused by the patient. | Weak |
Table 8.1: Urinary markers in the surveillance setting*
Marker | Sensitivity overall | HG | Specificity overall | HG | PPV overall | HG | NPV overall | HG | N studies/patients |
XPERT BC® MONITOR | 0.72 | 0.88 | 0.76 | 0.75 | 0.43 | 0.18 | 0.92 | 0.99 | 10/> 2000 |
EpiCheckTM | 0.74 | 0.91 | 0.84 | 0.81 | 0.48 | 0.43 | 0.94 | 0.98 | 5/1600 |
ADX BladderTM | 0.57 | 0.71 | 0.62 | 0.76 | 0.29 | 0.37 | 0.82 | 0.93 | 3/1600 |
CX BLADDER | 0.91 | - | 0.61 | - | 0.16 | - | 0.98 | - | 2/1000 |
FDFGR3+TERT | 0.93 | - | 0.79 | - | 0.67 | - | 0.96 | - | 2/250 |
- *Data extracted from a pooled analyses of systematic review [8].
- HG = high grade; NMIBC = non-muscle-invasive bladder cancer; PPV = positive predictive value; NPV = negative predictive value; n = number.