7. DISEASE MANAGEMENT
7.1. Localised low-risk disease
7.1.1. General considerations on kidney-sparing surgery
Kidney-sparing surgery for low-risk UTUC reduces the morbidity associated with radical surgery (e.g., loss of kidney function), without compromising oncological outcomes [200]. In low-risk cancers, it is the preferred approach as survival is similar to that after RNU [200]. This option should therefore be discussed in all low-risk cases, irrespective of the status of the contralateral kidney. Recommendations for kidney-sparing management of UTUC are listed in Section 7.1.7.
7.1.2. Ureteroscopy
Endoscopic ablation should be considered in patients with clinically low-risk cancer [201,202]. A flexible ureteroscope is useful in the management of pelvicalyceal tumours [203]. The patient should be informed of the need and be willing to comply with an early second-look URS [204] and stringent surveillance; complete tumour resection or destruction is necessary [204]. Nevertheless, a risk of disease progression remains with endoscopic management due to the suboptimal performance of imaging and biopsy for risk stratification and tumour biology [205].
7.1.3. Percutaneous access
Percutaneous management can be considered for low-risk UTUC in the renal pelvis [201,206] (LE: 3). This may also be offered for low-risk tumours in the lower caliceal system that are inaccessible or difficult to manage by flexible URS. However, this approach is being used less due to the availability of improved endoscopic tools such as distal-tip deflection of recent ureteroscopes [202,206]. Moreover, a risk of tumour seeding remains with percutaneous access [206].
7.1.4. Ureteral resection
Segmental ureteral resection with wide margins provides adequate pathological specimens for staging and grading while preserving the ipsilateral kidney. Lymphadenectomy can also be performed during segmental ureteral resection [200]. Segmental resection of the proximal two-thirds of ureter is associated with higher failure rates than for the distal ureter [207,208] (LE: 3).
Distal ureterectomy with ureteroneocystostomy are indicated for low-risk tumours in the distal ureter that cannot be removed completely endoscopically [190,207,209] (LE: 3). A total ureterectomy with an ileal-ureteral substitution or renal autotransplantation with pyelocystostomy is technically feasible, but only in selected cases when a renal-sparing procedure is mandatory and the tumour is low risk [210,211].
7.1.5. Chemo-ablation
A single-arm phase III trial showed that the use of mitomycin-containing reverse thermal gel (UGN-101) instillations in a chemoablation setting via retrograde catheter to the renal pelvis and calyces was associated with a complete response rate in a total of 42 patients (59%) with biopsy-proven low-grade UTUC measuring less than 15 mm. Fifty-six percent of patients remained in complete response after 12 months with Kaplan-Meier analysis of durability estimated as 82% [212]. The most frequently reported all-cause adverse events were ureteric stenosis in 31 (44%) of 71 patients, urinary tract infection in 23 (32%), haematuria in 22 (31%), flank pain in 21 (30%), and nausea in 17 (24%). A total of 19 (27%) of 71 patients had drug-related or procedure-related serious adverse events. No deaths were regarded as related to treatment [213].
7.1.6. Adjuvant instillations
7.1.6.1. Upper urinary tract
The antegrade instillation of BCG or mitomycin C in the upper urinary tract via percutaneous nephrostomy after complete tumour eradication has been studied for CIS after kidney-sparing management [176,214]
(LE: 3). Retrograde instillation through a single-J open-ended ureteric stent is also used. Both the antegrade and retrograde approach can be dangerous due to possible ureteric obstruction and consecutive pyelovenous influx during instillation/perfusion. The reflux obtained from a double-J stent has been used but this approach is suboptimal because the drug often does not reach the renal pelvis [215-218].
A systematic review and meta-analysis assessing the oncologic outcomes of patients with papillary UTUC or CIS of the upper tract treated with kidney-sparing surgery and adjuvant endocavitary treatment analysed the effect of adjuvant therapies (i.e., chemotherapeutic agents and/or immunotherapy with BCG) after kidney-sparing surgery for papillary non-invasive (Ta–T1) UTUCs and of adjuvant BCG for the treatment of upper tract CIS, finding no difference between the method of drug administration (antegrade vs. retrograde vs. combined approach) in terms of recurrence, progression, CSS, and OS. Furthermore, the recurrence rates following adjuvant instillations are comparable to those reported in the literature in untreated patients, questioning their efficacy [219]. The analyses were based on retrospective small studies suffering from publication and reporting bias.
Recent evidence suggests that early single adjuvant intracavitary upper tract instillation of mitomycin C in patients with low-grade UTUC might reduce the risk of local recurrence [220] (LE: 3). The authors report limited complications related to the instillations, but propose a retrograde pyelography before instillations are commenced to exclude contrast extravasation. This concept will need further evaluation in a randomised context [220].
7.1.6.2. Bladder
There is currently no data to support the use of bladder instillation of chemotherapy after kidney-sparing surgery as available RCTs included only patients who received RNU.
7.1.7. Recommendation for kidney-sparing management of localised low-risk UTUC
Recommendation | Strength rating |
Offer kidney-sparing management as primary treatment option to patients with low-risk tumours. | Strong |
7.2. Localised high-risk disease
7.2.1. Radical nephroureterectomy
7.2.1.1. Surgical approach
7.2.1.1.1. Open radical nephroureterectomy
Open RNU with bladder cuff excision is the standard treatment of high-risk UTUC, regardless of tumour location [24] (LE: 3). Radical nephroureterectomy must be performed according to oncological principles preventing tumour seeding [24]. Section 7.2.5 lists the recommendations for RNU.
7.2.1.1.2. Minimal invasive radical nephroureterectomy
Retroperitoneal metastatic dissemination and metastasis along the trocar pathway following manipulation of large tumours in a pneumoperitoneal environment have been reported in few cases [221,222]. Several precautions may lower the risk of tumour spillage:
1.avoid entering the urinary tract, except when performing a bladder cuff excision and only after prior clipping of the ureter and complete drainage of the bladder;
- avoid direct contact between instruments and the tumour;
- perform the procedure in a closed system. Avoid morcellation of the tumour and use an endobag for tumour extraction;
- the kidney and ureter must be removed en bloc with the bladder cuff;
- in invasive or large (T3/T4 and/or N+/M+) tumours an open approach is favoured, as the oncological outcomes may be better as compared to minimally-invasive RNU [223,224].
Laparoscopic RNU is safe in experienced hands when adhering to strict oncological principles. There is a tendency towards equivalent oncological outcomes after laparoscopic or open RNU [222,225-228] (LE: 3). One prospective randomised study has shown that laparoscopic RNU is inferior to open RNU for non-organ-confined UTUC. However, this was a small trial (n = 80) [224] (LE: 2). Oncological outcomes after RNU have not changed significantly over the past three decades despite staging and surgical refinements [229] (LE: 3). In a population-based data set, a hospital volume of > 6 patients per year treated with RNU showed improvement of short-term outcomes (30- and 90-day mortality) and overall long-term survival [230]. A robot-assisted laparoscopic approach can be considered with recent data suggesting oncologic equivalence with the other approaches [231-233]. In addition, robotic RNU can limit the risk of post-operative complication with shorter length of stay as compared to the laparoscopic approach [234].
7.2.1.1.3. Bladder cuff management
Resection of the distal ureter and its orifice is performed because there is a considerable risk of tumour recurrence in this area and in the bladder [195,207,235-237]. Several techniques have been considered to simplify distal ureter resection, including the pluck technique, stripping, transurethral resection of the intramural ureter, and intussusception. None of these techniques has convincingly been shown to be equal to complete bladder cuff excision [16,235,236] (LE: 3).
7.2.1.1.4. Lymph node dissection
The use of a LND template is likely to have a greater impact on patient survival than the number of removed LNs [238]. Template-based and completeness of LND improves CSS in patients with muscle-invasive disease and reduces the risk of local recurrence [239]. Even in clinically [240] and pathologically [241] node-negative patients, LND improves survival. The risk of LN metastasis increases with advancing tumour stage [162]. Lymph node dissection appears to be unnecessary in cases of TaT1 UTUC because of the low risk of LN metastasis [242-245], however, tumour staging is inaccurate pre-operatively; therefore a template-based LND should be offered to all patients who are scheduled for RNU for high-risk non-metastatic UTUC. The templates for LND have been described [239,246,247].
7.2.2. Distal ureterectomy
Distal ureterectomy for high-risk UTUC may be associated with similar oncological outcomes as compared to RNU [200]. This procedure can also provide the opportunity to perform a concomitant LN dissection. However, only selected cases of high-risk patients with distal ureter UTUC can benefit from this procedure, given the low level of evidence.
7.2.3. Kidney-sparing surgery for imperative indications
Kidney-sparing surgery, including ureterscopy or segmental ureterectomy, can be considered on a case-by-case basis for high-risk patients with imperative indications such as solitary kidney, bilateral UTUC, chronic kidney disease or any other comorbidity compromising the use of RNU (LE: 3). However, there is a greater risk of progression after kidney-sparing surgery for high- vs. low-risk UTUC with a direct impact on survival [200].
7.2.4. Peri-operative chemotherapy
7.2.4.1. Neoadjuvant treatments
7.2.4.1.1. Chemotherapy
The primary advantage of neoadjuvant chemotherapy (NAC) is the ability to give cisplatin-based regimens when patients still have maximal renal function. Several retrospective studies evaluating the role of NAC have shown evidence of pathological downstaging and complete response rates at RNU [170,248-251] with a direct impact on OS [183]. Furthermore, NAC has been shown to result in lower disease recurrence- and mortality rates compared to RNU alone, without compromising the use of definitive surgical treatment with a potential OS benefit [250,252-254].
No RCTs have been published yet but prospective data from a phase II trial showed that the use of NAC was associated with a 14% pathological complete response rate in high-grade UTUC [255]. In addition, final pathological stage was < ypT1 in more than 60% of included patients with acceptable toxicity profile. In a systematic review and meta-analysis comprising more than 800 patients, NAC has shown a pathologic partial response of 43% and a downstaging in 33% of patients, and also an OS and CSS survival benefit compared with RNU alone [256]. However, it is important to note that the evidence presented above is not conclusive, given the significant bias and heterogeneity of the available data.
7.2.4.1.2. Immunotherapy
Only a small phase II study including 10 patients with high-risk UTUC evaluated the efficacy of pembrolizumab in the neoadjuvant setting [257]. However, no pathological reponse was observed and one treatment-related death was reported. Thus, there is currently no evidence to support the use of neoadjuvant immunotherapy for high-risk UTUC.
7.2.4.2. Adjuvant treatments
7.2.4.2.1. Bladder instillations
The rate of bladder recurrence after RNU for UTUC is 22–47% [185,235]. Two prospective randomised trials [258,259] and two meta-analyses [260,261] have demonstrated that a single post-operative dose of intravesical chemotherapy (mitomycin C, pirarubicin) 2–10 days after surgery reduces the risk of bladder tumour recurrence within the first years post-RNU (LE: 2). Prior to instillation, a cystogram might be considered in case of any concerns about drug extravasation. All studies showed a very low risk of adverse events. Intravesical chemotherapy has also been safely given at the time of RNU, obviating the need for a post-operative cystogram, but with low level data for efficacy [262].
Based on current evidence it is unlikely that additional instillations beyond one peri-operative instillation of chemotherapy further substantially reduces the risk of intravesical recurrence [263]. Whilst there is no direct evidence supporting the use of intravesical instillation of chemotherapy after kidney-sparing surgery, single-dose chemotherapy might be effective in that setting as well (LE: 4). Management is outlined in Figures 7.1
and 7.2. One low-level evidence study suggested that bladder irrigation might reduce the risk of bladder recurrence after RNU [264].
7.2.4.2.2. Chemotherapy
A phase III multicentre prospective RCT (n = 261) evaluating the benefit of four cycles of adjuvant gemcitabine-platinum combination chemotherapy initiated within 90 days after RNU vs. surveillance has reported a significant improvement in disease-free survival (DFS) in patients with pT2–pT4, N (any) or LN-positive (pT any, N1–3) M0 UTUC [265] (LE: 1). Patients were stratified to gemcitabine/cisplatin or gemcitabine/carboplatin chemotherapy based on GFR alone.
The main potential limitation of using adjuvant chemotherapy is the concern that renal function may deteriorate after RNU. However, fractionated cisplatin may be considered to a GFR of 45 mL/min. The initial Galsky criteria defining cisplatinum eligibility (including GFR >/= 60 ml/min) are not routinely used outside of clinical trials across institutions [266,267]. In a retrospective study histological subtypes of UTUC exhibit different survival rates and adjuvant chemotherapy was only associated with an OS benefit in patients with pure UC [268] (LE: 3). However, whilst histological subtypes of UTUC exhibit different survival rates in retrospective studies, adjuvant chemotherapy should be considered where UC is the dominant pathology.
7.2.4.2.3. Immunotherapy
In a phase III, multicentre, double-blind RCT involving patients with high-risk muscle-invasive UC who had undergone radical surgery, adjuvant nivolumab improved DFS compared to placebo in the intention-to-treat population (20.8 vs. 10.8 months) and among patients with a programmed death-ligand 1 (PD-L1) expression level of 1% or more [269]. The patient population predominantly consisted of BC patients post-cystectomy, with an additional smaller cohort of patients with UTUC post-RNU. The median recurrence-free survival outside the urothelial tract in the entire intention-to-treat population was 22.9 months for nivolumab and 13.7 months for placebo. Treatment-related adverse events > grade 3 occurred in 17.9% of the nivolumab group and 7.2% of the placebo group. On subgroup analysis, patients with UTUC included in this study did not seem to benefit from adjuvant nivolumab, which requires further follow-up and analysis.
The European Medicines Agency (EMA) approved nivolumab as monotherapy for the adjuvant treatment of patients with muscle-invasive UC with tumour cell PD-L1 expression > 1%, who are at high risk of recurrence after undergoing radical surgery [270].
A network meta-analysis suggests superior oncological benefit for adjuvant platinum-based chemotherapy over immune checkpoint inhibitors in patients treated with radical surgery for UTUC [271].
7.2.4.2.4. Radiotherapy
Adjuvant radiation therapy has been suggested to control loco-regional disease after surgical removal. The data remains controversial and insufficient for conclusions [272-275]. Moreover, its added value to chemotherapy remains questionable [274].
7.2.5. Summary of evidence and recommendations for the management of high-risk non-metastatic UTUC
Summary of evidence | LE |
Radical nephroureterectomy is the standard treatment for high-risk UTUC, regardless of tumour location. | 2a |
Open, laparoscopic and robotic approaches have similar oncological outcomes for organ-confined UTUC. | 2a |
Failure to completely remove the bladder cuff increases the risk of BC recurrence. | 3 |
Lymphadenectomy improves survival in muscle-invasive UTUC. | 3 |
Post-operative platinum-based adjuvant chemotherapy improves disease-free survival. | 1b |
Single post-operative intravesical instillation of chemotherapy lowers the BC recurrence rate. | 1b |
Recommendations | Strength rating |
Perform radical nephroureterectomy (RNU) in patients with high-risk non-metastatic upper tract urothelial carcinoma (UTUC). | Strong |
Perform open RNU in non-organ confined UTUC. | Weak |
Perform a template-based lymphadenectomy in patients with high-risk non-metastatic UTUC. | Weak |
Offer adjuvant platinum-based chemotherapy after RNU to patients with pT2–T4 and/or pN+ disease. | Strong |
Deliver a post-operative bladder instillation of chemotherapy to lower the intravesical recurrence rate. | Strong |
Discuss adjuvant nivolumab with patients unfit for, or who declined, platinum-based adjuvant chemotherapy for > pT3 and/or pN+ disease after RNU alone or > ypT2 and/or ypN+ disease after neoadjuvant chemotherapy, followed by RNU. | Weak |
Offer distal ureterectomy to selected patients with high-risk tumours limited to the distal ureter. | Weak |
Offer kidney-sparing management to high-risk patients with imperative indication on a case-by-case basis, in consultation with the patient. | Strong |
Figure 7.1: Proposed flowchart for the management of UTUC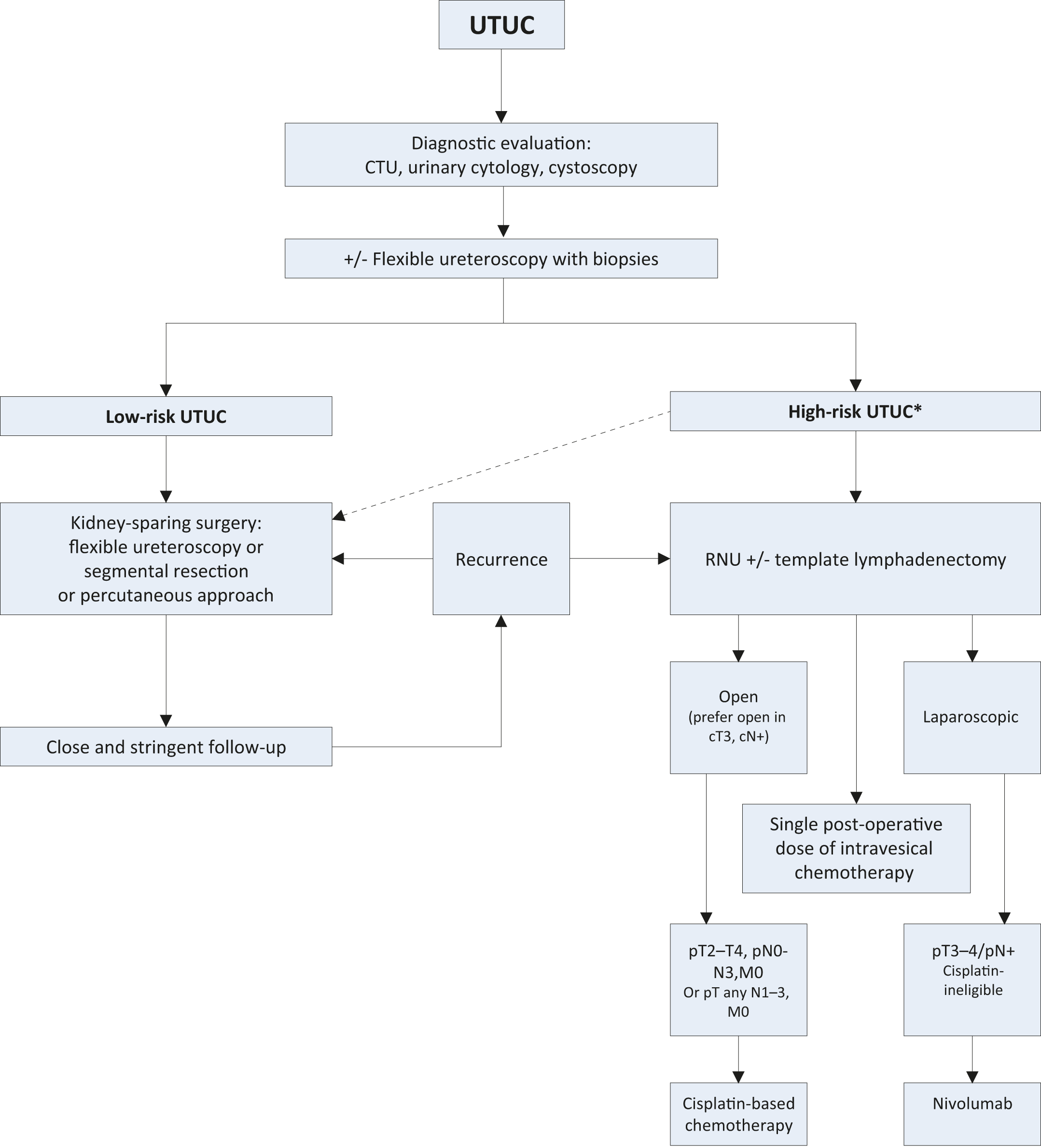 *In patients with solitary kidney, consider a more conservative approach.
*In patients with solitary kidney, consider a more conservative approach.
CTU = computed tomography urography; RNU = radical nephroureterectomy; UTUC = upper urinary tract urothelial carcinoma.
Figure 7.2: Surgical treatment according to location and risk status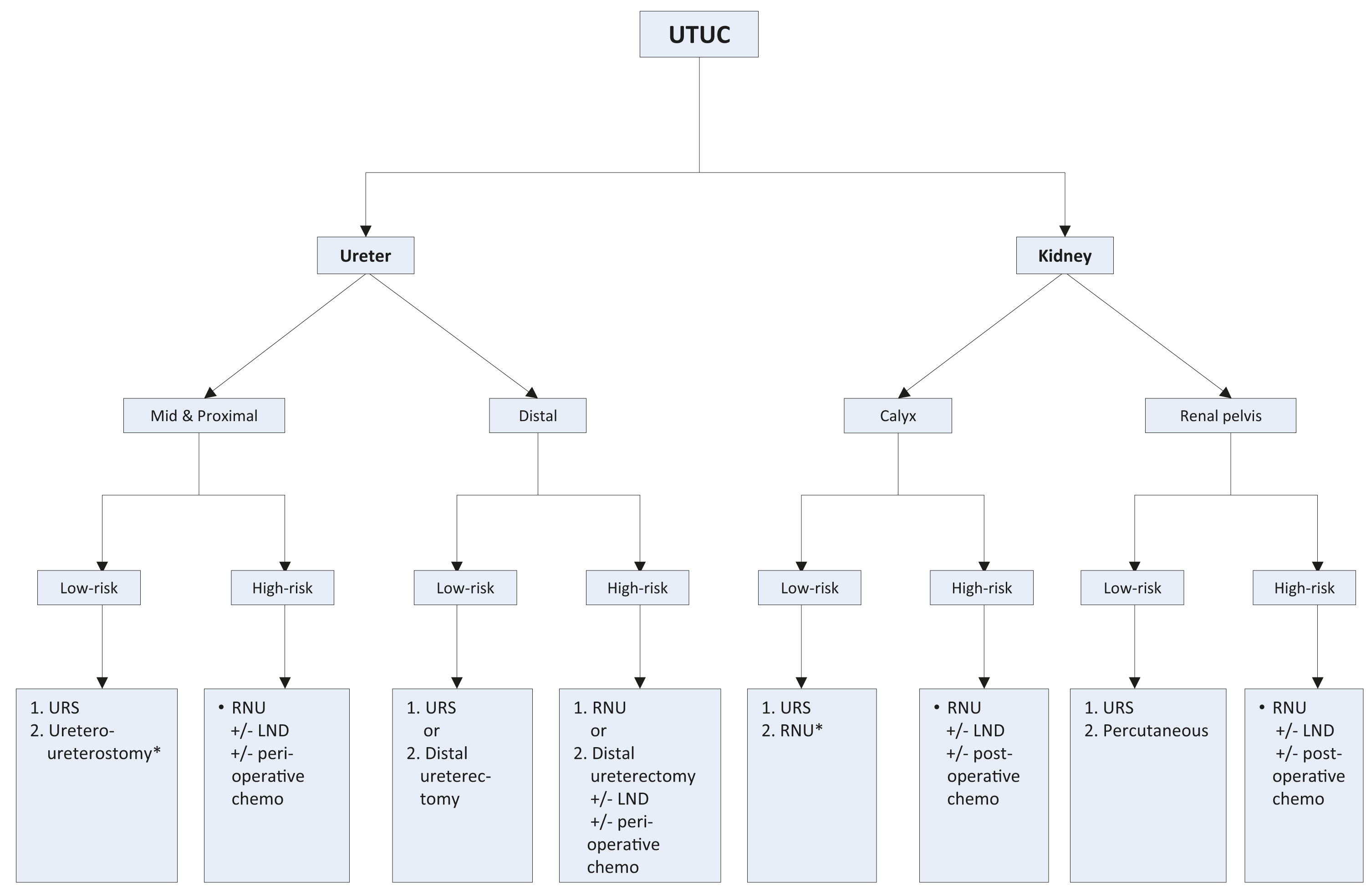 1 = first treatment option; 2 = secondary treatment option.
1 = first treatment option; 2 = secondary treatment option.
*In case not amendable to endoscopic management.LND = lymph node dissection; RNU = radical nephroureterectomy; URS = ureteroscopy; UTUC = upper urinary tract urothelial carcinoma.
7.3. Metastatic disease
7.3.1. Clinical loco-regional lymph node metastases
Evidence is lacking regarding the optimal management of clinical node-positive disease. Patients with clinically N+ UTUC should be offered first-line chemotherapy. In patients whose cancer responds or who have stable disease, maintenance avelumab can be offered [276]. Depending on the extent of the nodal disease (i.e., cN1/N2) surgical resection with LN dissection can be discussed after initial systemic therapy. In patients whose cancer progress, second-line treatment can be offered, similar to metastatic disease [277,278].
7.3.2. Distant metastases
7.3.2.1. Systemic treatments
7.3.2.1.1. First-line setting
7.3.2.1.1.1. Patients fit for cisplatin-based combination chemotherapy
Upper tract UC and urothelial BC both respond to systemic platinum-based chemotherapy. A retrospective analysis of three RCTs showed that primary tumour location in the lower- or upper urinary tract had no impact on progression-free survival (PFS) or OS in patients with locally-advanced or metastatic UC treated with platinum-based combination chemotherapy [279]. Therefore, cisplatin-containing combination chemotherapy is the standard treatment for advanced or metastatic UTUC [2]. A number of cisplatin-containing chemotherapy regimens have proven efficacy although gemcitabine and cisplatin are the most widely used. The use of cisplatin-based chemotherapy is widely considered in patients with eGFR > 45 mL/min [279].
The efficacy of immunotherapy using PD1 or PD-L1 inhibitors has been evaluated in the first-line setting for the treatment of cisplatin/carboplatin-fit patients with metastatic UC, including those with UTUC [280]. First-line immune checkpoint inhibitors or the combination of platinum-based chemotherapy with immune checkpoint inhibitors have not resulted in positive significant survival advantages and are not currently recommended
[281-283].
7.3.2.1.1.2. Patients unfit for cisplatin-based combination chemotherapy
Carboplatin-based chemotherapy is recommended in patients unfit for cisplatin [2]. Carboplatin with gemcitabine is the preferred regimen [284], irrespective of PDL-1 status. In a recent critical re-analysis of RCTs comparing OS after cisplatin vs. carboplatin-based regimens in advanced UC, cisplatin confered a minor OS benefit compared to carboplatin [285].
7.3.2.1.1.3. Maintenance therapy after first-line platinum-based chemotherapy
Maintenance avelumab is recommended in patients with complete/partial response or stable disease after 4–6 cycles of platinum-based chemotherapy. Data from a phase III RCT showed that the use of avelumab maintenance therapy after 4 to 6 cycles of gemcitabine plus cisplatin or carboplatin (started within 10 weeks of completion of first-line platinum-based chemotherapy) significantly prolonged OS as compared to best supportive care alone in those patients with advanced or metastatic UC who did not progress during, or responded to, first-line chemotherapy (HR: 0.69; 95% CI: 0.56–0.86) [276,286]. An increase in median OS from 14 to 21 months was observed with avelumab. Although no subgroup analysis based on tumour location was available in this study, almost 30% of the included patients had UTUC. Similarly, in a phase II study comprising 108 patients with metastatic UC achieving at least stable disease on first-line platinum-based chemotherapy, maintenance pembrolizumab improved PFS compared to placebo (5.4 vs. 3.0 months) [287].
7.3.2.1.1.4. Patients unfit for platinum-based combination chemotherapy
Pembrolizumab or atezolizumab are alternative choices for patients who are PD-L1 positive and not eligible/fit for platinum-based chemotherapy. In a single-arm phase II trial (n = 370) of cisplatin-ineligible UC, pembrolizumab monotherapy was associated with an objective response rate of 26% in 69 metastatic UTUC patients [288]. In the overall cohort, a PD-L1 expression of 10% was associated with a greater response rate to pembrolizumab. Treatment-related toxicity was in line with previous studies. In a single-arm phase II trial(n = 119) of cisplatin-ineligible UC, atezolizumab monotherapy was associated with an objective response rate of 39% in 33 (28%) metastatic UTUC patients [289]. Median OS in the overall cohort was 15.9 months and treatment-related toxicity was in line with previous studies [282].
7.3.2.1.2. Second-line setting
7.3.2.1.2.1. Immunotherapy
A phase III RCT including 542 patients who received prior platinum-based chemotherapy for advanced UC showed that pembrolizumab decreased the risk of death compared to second-line chemotherapy (the investigator’s choice of paclitaxel, docetaxel, or vinflunine); median OS: 10.3 months for pembrolizumab and 7.4 months for chemotherapy (HR: 0.73; 95% CI: 0.59–0.91) [290]. Responses were more frequent and durable for pembrolizumab compared with chemotherapy (21% vs. 11%). In the UTUC subgroup (n = 75/13.8%), the OS benefit seemed larger (50%).
The IMVigor211 trial explored atezolizumab in PD-L1 biomarker-positive tumours in patients with tumours which relapsed after platinum-based chemotherapy and failed to show a significant OS advantage [291].
Other immunotherapies such as nivolumab [292], avelumab [293,294] and durvalumab [295] have shown objective response rates ranging from 17.8% [295] to 19.6% [292] and median OS ranging from 7.7 months to 18.2 months in patients with platinum-resistant metastatic UC. These results were obtained from single-arm phase I or II trials only and the number of UTUC patients included in these studies was only specified for avelumab (n = 7/15.9%) without any subgroup analysis based on primary tumour location [294].
The immunotherapy combination of nivolumab plus ipilimumab has shown significant anti-tumour activity with objective response rate up to 38% in a phase I/II multicentre trial including 78 patients with metastatic UC progressing after platinum-based chemotherapy [296]. Although UTUC patients were included in this trial, no subgroup analysis was available. Other immunotherapy combinations may be effective in the second-line setting but data are currently limited [297].
7.3.2.1.2.2. Novel agents
7.3.2.1.2.2.1.Fibroblast growth factor receptors (FGFR) inhibition
Erdafitinib, a pan-FGFR tyrosine kinase inhibitor of FGFR1–4, was associated with a 40% radiological response rate (RECIST) in a phase II trial of 99 patients with locally-advanced or metastatic UC who progressed after first-line chemotherapy and harboured a FGFR DNA genomic alterations (FGFR2/3 mutations or FGFR3 fusions) [298]. This study included 23 UTUC patients with visceral metastases showing a 43% radiological response rate. No OS data are available to date.
7.3.2.1.2.2.2.Antibody drug conjugates (ADC)
A phase II study enrolled 89 patients (of whom 43% had UTUC) with cisplatin-unfit metastatic UC progressing after therapy with PD-1 or PD-L1 inhibitors. All patients received the antibody–drug conjugate enfortumab vedotin. The objective radiological response rate (RECIST) was 52% of which 20% of patients achieved complete response [299]. In a phase III trial of enfortumab vedotin for the treatment of patients with locally-advanced or metastatic UC who had previously received platinum-containing chemotherapy and had disease progression during or after treatment with a PD-1 or PD-L1 inhibitor, enfortumab vedotin significantly prolonged survival as compared with standard chemotherapy (median OS 12.88 vs. 8.97 months) [300].
7.3.2.1.3. Third-line setting
In an open-label phase II trial a total of 108 patients with metastatic UC who progressed after platinum-based chemotherapy and checkpoint inhibitors were treated with the antibody-drug conjugate sacituzumab govitecan. The objective radiological response rate was 27%, with median duration of response of 7.2 months, median PFS of 5.4 months and median OS of 10.9 months. However, the proportion of patients with UC was not mentioned in the publication [301].
A pre-planned subgroup analysis from the phase III RANGE trial assessed the impact on outcomes and safety of ramucirumab added to docetaxel after disease progression on both platinum-based chemotherapy and immune checkpoint inhibitors [302]. Median PFS was 3.15 months on ramucirumab/docetaxel vs. 2.73 months on placebo/docetaxel (HR: 0.786; 95% CI: 0.404–1.528, p = 0.4877). This trend for ramucirumab benefit occurred despite the ramucirumab arm having a higher percentage of patients with poorer prognosis. However, these findings need confirmation by further studies, as this analysis is limited by patient numbers and an imbalance in the treatment arms.
7.3.2.2. Surgery
7.3.2.2.1. Radical nephroureterectomy
Data regarding RNU in the metastatic setting are lacking with mainly retrospective observational studies [303-305].
Although evidence remains very limited, RNU may be associated with CSS [304,306,307] and OS benefit in selected patients, especially those fit enough to receive cisplatin-based chemotherapy [303,304]. It is noteworthy that these benefits may be limited to those patients with only one metastatic site [304]. Nonetheless, given the high risk of bias of the observational studies addressing RNU for metastatic UTUC, indications for RNU in this setting should mainly be reserved for palliative patients, aimed at controlling symptomatic disease [20,118] (LE: 3).
7.3.2.2.2. Metastasectomy
There is no UTUC-specific study supporting the role of metastasectomy in patients with advanced disease. Reports suggesting that resection of metastatic lesions could be safe and oncologically beneficial in selected patients should be interpreted with caution [308-312]. In the absence of data from RCTs, patients should be evaluated on an individual basis and the decision to perform a metastasectomy (surgically) should be made following a shared decision-making process with the patient (LE: 3).
7.3.3. Summary of evidence and recommendations for the treatment of metastatic UTUC
Summary of evidence | LE |
Cisplatin-based combination chemotherapy can improve median survival. | 2 |
Cisplatin-containing combination chemotherapy is the standard of care in advanced or metastatic patients fit enough to tolerate cisplatin. | 1b |
Carboplatin-based combination chemotherapy offers a survival benefit in cisplatin unfit patients. | 1b |
Non-platinum combination chemotherapy has not been tested against standard chemotherapy in patients who are fit or unfit for cisplatin combination chemotherapy. | 4 |
Maintenance avelumab is associated with an OS advantage compared with best supportive care in patients who did not have disease progression after 4 to 6 cycles of gemcitabine plus either cisplatin or carboplatin. | 1b |
PD-1 inhibitor pembrolizumab has been approved for patients who have progressed during or after previous platinum-based chemotherapy and did not receive previous immune therapy based on the results of a phase III trial. | 1b |
PD-L1 inhibitor atezolizumab has been approved for patients that have progressed during or after previous platinum-based chemotherapy and did not receive previous immune therapy based on the results of a phase II trial. | 2a |
PD-1 inhibitor nivolumab has been approved for patients whose disease has progressed during or after previous platinum-based chemotherapy and did not receive previous immune therapy based on the results of a phase II trial. | 2a |
PD-1 inhibitor pembrolizumab has been approved for patients with advanced or metastatic UC unfit for platinum-based first-line chemotherapy based on the results of a phase II trial but use of pembrolizumab is restricted to PD-L1 positive patients. | 2a |
PD-L1 inhibitor atezolizumab has been approved for patients with advanced or metastatic UC unfit for platinum-based first-line chemotherapy based on the results of a phase II trial, but use of atezolizumab is restricted to PD-L1 positive patients. | 2a |
Erdafitinib was associated with radiological response in platinum-refractory patients with locally-advanced or metastatic UC and FGFR DNA genomic alterations (FGFR2/3 mutations or FGFR3 fusions). | 2a |
Enfortumab vedotin was associated with OS benefit in patients who had previously received platinum-containing chemotherapy and experienced disease progression during or after treatment with a PD-1 or PD-L1 inhibitor. | 1b |
Palliative nephroureterectomy can improve quality of life by controlling symptomatic disease. | 3 |
RNU can confer a survival benefit in highly selected patients. | 4 |
Recommendations | Strength rating |
First-line treatment for platinum-eligible patients | |
Offer platinum combination chemotherapy to platinum-eligible patients. | Strong |
Offer cisplatin-based chemotherapy with gemcitabine/cisplatin or HD-MVAC to cisplatin-eligible patients. | Strong |
Offer maintenance avelumab to patients who did not have disease progression after 4 to 6 cycles of gemcitabine plus cisplatin/carboplatin. | Strong |
First-line treatment in patients ineligible for cisplatin or carboplatin | |
Offer gemcitabine/carboplatin chemotherapy to cisplatin-ineligible patients. | Strong |
Offer checkpoint inhibitors pembrolizumab or atezolizumab to patients with PD-L1 positive tumours. | Weak |
Second-line treatment | |
Offer checkpoint inhibitor (pembrolizumab) to patients with disease progression during or after platinum-based combination chemotherapy for metastatic disease. | Strong |
Offer enfortumab vedotin to patients previously treated with platinum-containing chemotherapy and who had disease progression during or after treatment with a PD-1 or PD-L1 inhibitor. | Strong |
Only offer vinflunine to patients for metastatic disease as second-line treatment if immunotherapy or combination chemotherapy is not feasible. Alternatively, offer vinflunine as third- or subsequent-line treatment. | Strong |
Offer erdafitinib as subsequent-line therapy to platinum-refractory patients with FGFR DNA genomic alterations (FGFR2/3 mutations or FGFR3 fusions). | Weak |
Offer nephroureterectomy as a palliative treatment to symptomatic patients with resectable locally-advanced tumours. | Weak |
DNA = deoxyribonucleic acid; FGFR = fibroblast growth factor receptors; HD-MVAC = high-dose intensitymethotrexate, vinblastine, adriamycin plus cisplatin; PD-L1 = programmed death ligand 1.