1. INTRODUCTION
1.1. Aim and scope
The European Association of Urology (EAU) Non-muscle-invasive Bladder Cancer (NMIBC) Guidelines Panel has compiled these clinical guidelines to provide clinicians with evidence-based information and recommendations for the management of upper urinary tract urothelial carcinoma (UTUC). Separate EAU guidelines are available addressing non-muscle-invasive bladder cancer [1], muscle-invasive and metastatic bladder cancer (MIBC) [2], and primary urethral carcinoma [3].
It must be emphasised that clinical guidelines present the best evidence available to the experts but following guideline recommendations will not necessarily result in the best outcome. Guidelines can never replace clinical expertise when making treatment decisions for individual patients, but rather help to focus decisions - also taking personal values and preferences/individual circumstances of patients into account. Guidelines are not mandates and do not purport to be a legal standard of care.
1.2. Panel composition
The European Association of Urology (EAU) Guidelines Panel on NMIBC consists of an international multidisciplinary group of clinicians, including urologists, uro-oncologists, a radiologist, a pathologist, and a statistician. Members of this panel have been selected based on their expertise and to represent the professionals treating patients suspected of harbouring urothelial carcinoma (UC). In the course of 2021 two patient representatives have formally joined the NMIBC Panel. All involved in the production of this document have submitted potential conflict of interest statements, which can be viewed on the EAU website Uroweb: https://uroweb.org/guideline/upper-urinary-tract-urothelial-cell-carcinoma/.
1.3. Available publications
A quick reference document (Pocket guidelines) is available presenting the main findings of the UTUC Guidelines. This is an abridged version which may require consultation together with the full text version. Several scientific publications are available as are a number of translations of all versions of the EAU UTUC Guidelines. The most recent scientific summary was published in 2021 [4]. All documents are accessible through the EAU website: https://uroweb.org/guideline/upper-urinary-tract-urothelial-cell-carcinoma/.
1.4. Publication history & summary of changes
The first EAU Guidelines on UTUC were published in 2011. This 2023 publication presents a limited update of the 2022 version.
1.4.1. Summary of changes
The literature for the complete document has been assessed and updated, whenever relevant. Conclusions and recommendations have been rephrased and added to throughout the current document.
Key changes for the 2023 print can be found in:
- Section 5.7 Summary of evidence and recommendations for the diagnosis of UTUC, the following recommendations were revised:
Recommendations | Strength rating |
2022 recommendation: Use diagnostic ureteroscopy and biopsy if imaging and cytology are not sufficient for the diagnosis and/or risk-stratification of the tumour. | Strong |
Revised 2023 recommendation: Use diagnostic ureteroscopy (preferably without biopsy) if imaging and/or voided urine cytology are not sufficient for the diagnosis and/or risk-stratification of patients suspected to have UTUC. | Strong |
2022 recommendation: Magnetic resonance urography or 18F-Fluorodeoxglucose positron emission tomography/CT may be used when CT is contra-indicated. | Weak |
Revised 2023 recommendation: Magnetic resonance urography or 18F-Fluorodeoxglucose positron emission tomography/CT (to assess [nodal] metastasis) may be used when CT is contra-indicated. | Weak |
- Chapter 7 Disease Management has been restructured resulting in recommendations moved to other sections and new recommendations having been added to Section 7.2.5 Summary of evidence and guidelines for the management of high-risk non-metastatic UTUC:
Summary of evidence | LE |
Post-operative platinum-based adjuvant chemotherapy improves disease-free survival. | 1b |
Recommendations | Strength rating |
New 2023 recommendation: Offer adjuvant platinum-based chemotherapy after RNU to patients with pT2–T4 and/or pN+ disease. | Strong |
New 2023 recommendation: Discuss adjuvant nivolumab with patients unfit for, or who declined, platinum-based adjuvant chemotherapy for > pT3 and/or pN+ disease after RNU alone or > ypT2 and/or ypN+ disease after neoadjuvant chemotherapy, followed by RNU. | Weak |
2022 recommendation: Offer kidney-sparing management to patients with solitary kidney and/or impaired renal function, providing that it will not compromise survival. This decision will have to be made on a case-by-case basis in consultation with the patient. | Strong |
Revised 2023 recommendation: Offer kidney-sparing management to high-risk patients with imperative indication on a case-by-case basis, in consultation with the patient. | Strong |
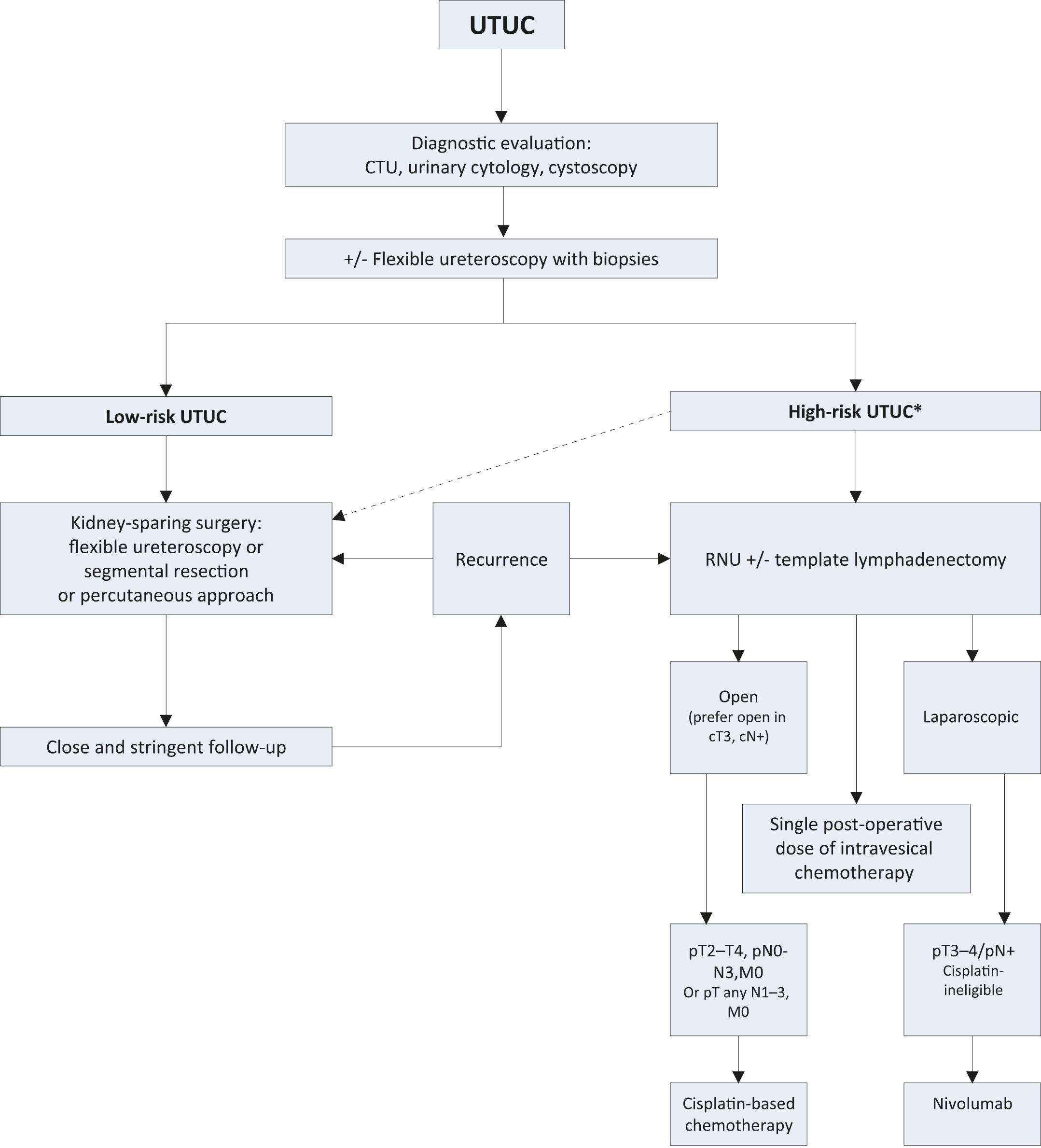
- New data prompted changes to Figure 7.1 Proposed flowchart for the management of UTUC.
- Section 7.3.3 Summary of evidence and recommendations for the treatment of metastatic UTUC:
Summary of evidence | LE |
Carboplatin-based combination chemotherapy offers a survival benefit in cisplatin unfit patients. | 1b |
Erdafitinib was associated with radiological response in platinum-refractory patients with locally-advanced or metastatic UC and FGFR DNA genomic alterations (FGFR2/3 mutations or FGFR3 fusions). | 2a |
Enfortumab vedotin was associated with OS benefit in patients who had previously received platinum-containing chemotherapy and experienced disease progression during or after treatment with a PD-1 or PD-L1 inhibitor. | 1b |
Palliative nephroureterectomy can improve quality of life by controlling symptomatic disease. | 3 |
RNU can confer a survival benefit in highly selected patients. | 4 |
Recommendations | Strength rating |
First-line treatment for cisplatin-eligible patients | |
New 2023 recommendation: Offer platinum combination chemotherapy to platinum-eligible patients. | Strong |
First-line treatment in patients ineligible for cisplatin or carboplatin | |
New 2023 recommendation: Offer gemcitabine/carboplatin chemotherapy to cisplatin-ineligible patients. | Strong |
2022 recommendation: Offer checkpoint inhibitors pembrolizumab or atezolizumab depending on PD-L1 status. | Weak |
Revised 2023 recommendation: Offer checkpoint inhibitors pembrolizumab or atezolizumab to patients with PD-L1 positive tumours. | Weak |
Second-line treatment | |
New 2023 recommendation: Offer enfortumab vedotin to patients previously treated with platinum-containing chemotherapy and who had disease progression during or after treatment with a PD-1 or PD-L1 inhibitor. | Strong |
2022 recommendation: Offer erdafitinib in platinum-refractory tumours with FGFR alterations. | Strong |
Revised 2023 recommendation: Offer erdafitinib as subsequent-line therapy to platinum-refractory patients with FGFR DNA genomic alterations (FGFR2/3 mutations or FGFR3 fusions). | Weak |
DNA = deoxyribonucleic acid; FGFR = fibroblast growth factor receptors; PD-L1 = programmed death ligand 1.
- Section 8.1 Summary of evidence and recommendations for the follow-up of UTUC:
Recommendations | Strength rating |
After kidney-sparing management | |
Low-risk tumours | |
2022 recommendation: Perform ureteroscopy (URS) at 3 months. | Weak |
Revised 2023 recommendation: Perform ureteroscopy (URS) at 3 months if no second-look ureteroscopy was performed. | Weak |