3. EPIDEMIOLOGY AETIOLOGY AND PATHOLOGY
3.1. Epidemiology
Urothelial carcinomas are the sixth most common tumours in developed countries [9]. They can be localised in the lower (bladder and urethra) and/or the upper (pyelocaliceal cavities and ureter) urinary tract. Bladder tumours account for 90–95% of UCs and are the most common urinary tract malignancy [1]. Upper urinary tract UCs are uncommon and account for only 5–10% of UCs [9] with an estimated annual incidence in Western countries of almost two cases per 100,000 inhabitants. This rate has risen in the past few decades as a result of improved detection and improved bladder cancer (BC) survival [10,11]. Pyelocaliceal tumours are approximately twice as common as ureteral tumours and multifocal tumours are found in approximately 10–20% of cases [12]. The presence of concomitant carcinoma in situ of the upper tract is between 11% and 36% [10]. In 17% of cases, concurrent BC is present [13] whilst a prior history of BC is found in 41% of American men but in only 4% of Chinese men [14]. This, along with genetic and epigenetic factors, may explain why Asian patients present with more advanced and higher-grade disease compared to other ethnic groups [10]. Following treatment, recurrence in the bladder occurs in 22–47% of UTUC patients, depending on initial tumour grade [15] compared with 2–5% in the contralateral upper tract [16]. A retrospective international registry including data from 2,380 patients from 2014 to 2019 (101 centres in 29 countries) confirmed that UTUC patients were predominantly male (70.5%) and 53.3% were past or present smokers. The majority of patients (58.1%) were evaluated because of symptoms, mainly visible haematuria [17]. The latter was confirmed by a meta-analysis pooling 44 studies and showing a pooled incidence rate for UTUC of 0.75% for visible haematuria and 0.17% for nonvisible haematuria [18].
With regards to UTUC occurring following an initial diagnosis of BC, a series of 82 patients treated with bacillus Calmette-Guérin (BCG) who had regular upper tract imaging between years 1 and 3 showed a 13% incidence of UTUC, all of which were asymptomatic [19], whilst in another series of 307 patients without routine upper tract imaging the incidence was 25% [20]. A multicentre cohort study (n = 402) with a 50 month follow-up demonstrated a UTUC incidence of 7.5% in NMIBC patients receiving BCG with predictors being intravesical recurrence and non-papillary tumour at transurethral resection of the bladder (TURB) [21]. Following radical cystectomy for MIBC, 3–5% of patients develop a metachronous UTUC [22,23].
Approximately two-thirds of patients who present with UTUCs have invasive disease at diagnosis compared to 15–25% of patients presenting with muscle-invasive bladder tumours [24]. This is probably due to the absence of muscularis propria layer in the upper tract, so tumours are more likely to upstage at an earlier time-point. Approximately 9% of patients present with metastasis [10,25-27]. Upper urinary tract UCs have a peak incidence in individuals aged 70–90 years and are twice as common in men [28].
Upper tract UC and BC exhibit significant differences in the prevalence of common genomic alterations. In individual patients with a history of both tumours, BC and UTUC are often clonally related. Genomic characterisation of UTUC provides information regarding the risk of bladder recurrence and can identify tumours associated with Lynch syndrome [29].
The Amsterdam criteria are a set of diagnostic criteria to help identify families which are likely to have Lynch syndrome [30]. In Lynch-related UTUC, immunohistochemistry (IHC) analysis showed loss of protein expression corresponding to the disease-predisposing MMR (mismatch repair) gene mutation in 98% of the samples (46% were microsatellite instable and 54% microsatellite stable) [31]. The majority of tumours develop in MSH2 and MSH6 mutation carriers [31]. Patients identified at high risk for Lynch syndrome should undergo germline DNA sequencing for patient and family counselling [32,33]. Germline mutations in DNA MMR genes defining Lynch syndrome are found in 9% of patients with UTUC compared to 1% of patients with BC, linking UTUC to Lynch syndrome [34]. A study of 115 consecutive UTUC patients, reported that 13.9% screened positive for potential Lynch syndrome and 5.2% had confirmed Lynch syndrome [35]. This is one of the highest rates of undiagnosed genetic disease in urological cancers, which justifies screening of all patients under 60 presenting with UTUC and those with a family history of UTUC (see Figure 3.1) [36,37] or positive reflexive MMR-test by IHC in sporadic UTUC [34,38-41].
Figure 3.1: Selection of patients with UTUC for Lynch syndrome screening during the first medical interview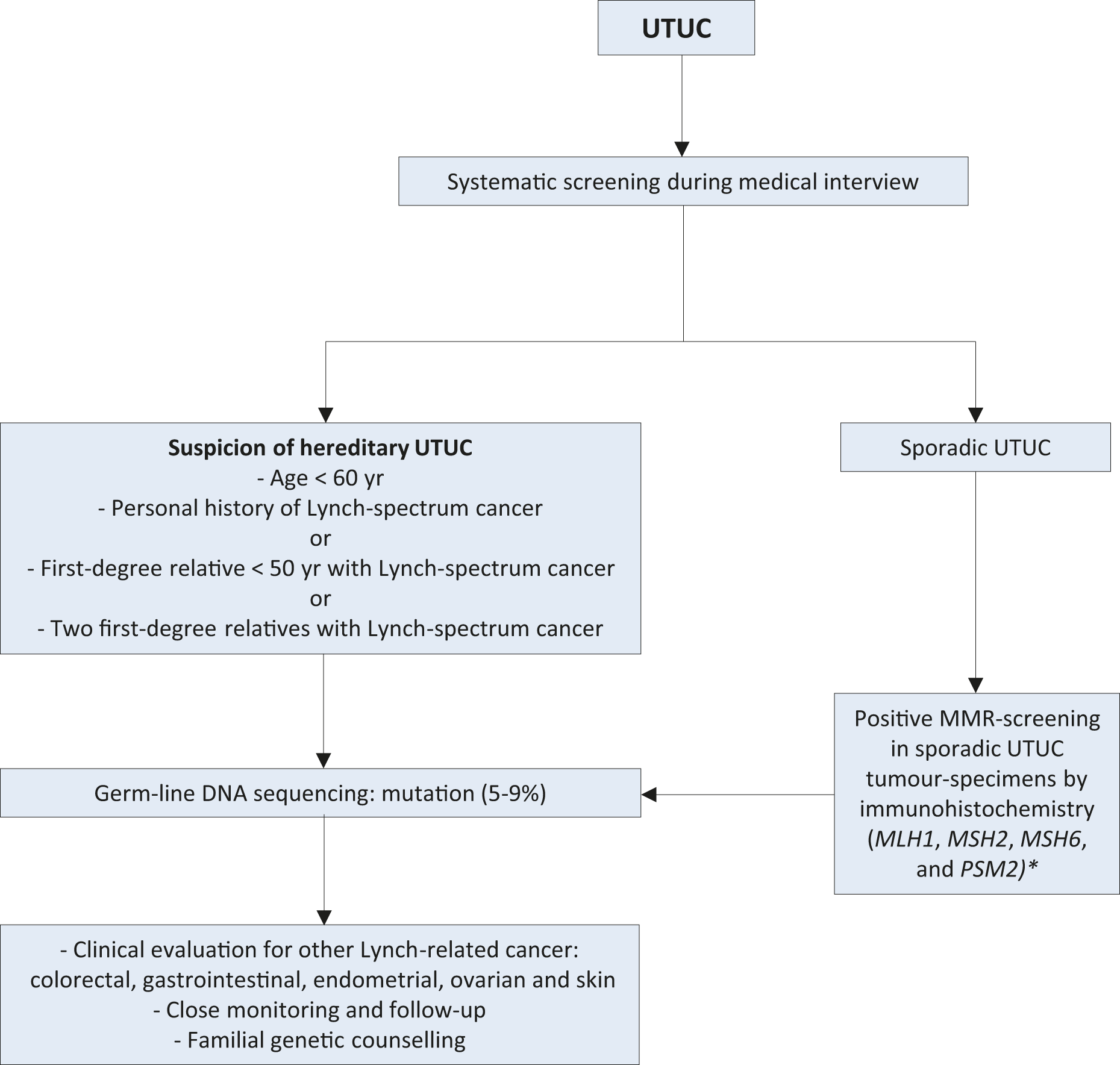 *Sporadic UTUC that for any reason has undergone MMR screening with a positive result should prompt subsequent testing for germline DNA sequencing mutations.MMR = mismatch repair; mismatch repair genes = MLH1, MSH2, MSH6, and PSM2; UTUC = upper urinary tract urothelial carcinoma.
*Sporadic UTUC that for any reason has undergone MMR screening with a positive result should prompt subsequent testing for germline DNA sequencing mutations.MMR = mismatch repair; mismatch repair genes = MLH1, MSH2, MSH6, and PSM2; UTUC = upper urinary tract urothelial carcinoma.
3.2. Risk factors
A number of environmental factors have been implicated in the development of UTUC [12,42]. Published evidence in support of a causative role for these factors is not strong, with the exception of smoking and aristolochic acid. Tobacco exposure increases the relative risk of developing UTUC from 2.5 to 7.0 [43-45]. A large population-based study assessing familial clustering in relatives of UC patients, including 229,251 relatives of case subjects and 1,197,552 relatives of matched control subjects, has demonstrated genetic or environmental roots independent of smoking-related behaviours. With more than 9% of the cohort being UTUC patients, clustering was not seen in upper tract disease. This may suggest that the familial clustering of UC is specific to lower tract cancers [46].
In Taiwan and Chile, the presence of arsenic in drinking water has been tentatively linked to UTUC [47,48].
Aristolochic acid, a nitrophenanthrene carboxylic acid produced by aristolochia plants, which are used worldwide, especially in China and Taiwan [49], exerts multiple effects on the urinary system. Aristolochic acid irreversibly injures renal proximal tubules resulting in chronic tubulointerstitial disease, while the mutagenic properties of this chemical carcinogen lead predominantly to UTUC [49-51]. Aristolochic acid has been linked to BC, renal cell carcinoma, hepatocellular carcinoma, and intrahepatic cholangiocarcinoma [52]. Two routes of exposure to aristolochic acid are known: (i) environmental contamination of agricultural products by aristolochia plants, as reported for Balkan endemic nephropathy [53]; and (ii) ingestion of aristolochia-based herbal remedies [54,55]. Following bioactivation, aristolochic acid reacts with genomic DNA to form aristolactam-deoxyadenosine adducts [56]; these lesions persist for decades in target tissues, serving as robust biomarkers of exposure [9]. These adducts generate a unique mutational spectrum, characterised by A>T transversions located predominately on the non-transcribed strand of DNA [52,57]. However, fewer than 10% of individuals exposed to aristolochic acid develop UTUC [51].
Two retrospective series found that aristolochic acid-associated UTUC is more common in females [58,59]. However, females with aristolochic acid UTUC have a better prognosis than their male counterparts. Consumption of arsenic in drinking water and aristolochia-based herbal remedies together appears to have an additive carcinogenic effect [60].
Alcohol consumption is associated with development of UTUC. A large case-control study (1,569 cases and 506,797 controls) has evidenced a significantly higher risk of UTUC in ever drinkers compared to never drinkers (OR: 1.23; 95% CI: 1.08–1.40, p = 0.001). Compared to never drinkers, the risk threshold for UTUC was > 15 g of alcohol/day. A dose-response was observed [61].
Differences in the ability to counteract carcinogens may contribute to host susceptibility to UTUC. Some genetic polymorphisms are associated with an increased risk of cancer or faster disease progression that introduces variability in the inter-individual susceptibility to the risk factors previously mentioned. Upper urinary tract UCs may share some risk factors and described molecular pathways with bladder UC [29]. So far, two UTUC-specific polymorphisms have been reported [62].
A history of BC is associated with a higher risk of developing UTUCs (see Section 3.1). Patients requiring ureteral stenting at the time of TURB, including prior to radical cystectomy, have been shown to have a higher risk for upper tract recurrence [63,64].
3.3. Histology and classification
3.3.1. Histological types
Upper urinary tract tumours are almost always UCs and pure non-urothelial histology is rare [65,66]. However, histological subtypes are present in approximately 25% of UTUCs [67,68]. Pure squamous cell carcinoma of the urinary tract is often assumed to be associated with chronic inflammatory diseases and infections arising from urolithiasis [69,70]. Urothelial carcinoma with divergent squamous differentiation is present in approximately 15% of cases [69]. Keratinising squamous metaplasia of urothelium is a risk factor for squamous cell cancers and therefore mandates surveillance. Upper urinary tract UCs with different subtypes are high-grade and have a worse prognosis compared with pure UC [68,71,72]. Other subtypes, although rare, include sarcomatoid and UCs with inverted growth [72].
However, collecting duct carcinomas, which may seem to share similar characteristics with UCs, display a unique transcriptomic signature similar to renal cancer, with a putative cell of origin in the distal convoluted tubules. Therefore, collecting duct carcinomas are considered as renal tumours [73].
3.4. Summary of evidence and recommendations for epidemiology, aetiology, and pathology
Summary of evidence | LE |
Aristolochic acid and smoking exposure increases the risk for UTUC. | 2a |
Patients with Lynch syndrome are at risk for UTUC. | 2a |
Recommendations | Strength rating |
Evaluate patient and family history based on the Amsterdam criteria to identify patients with upper tract urothelial carcinoma. | Weak |
Evaluate patient exposure to smoking and aristolochic acid. | Weak |