6. DISORDERS OF EJACULATION
6.1. Introduction
Ejaculation is a complex physiological process that comprises emission and expulsion processes and is mediated by interwoven neurological and hormonal pathways [678]. Any interference with those pathways may cause a wide range of ejaculatory disorders (Table 16).
Table 16: Spectrum of ejaculation disorders
Spectrum of ejaculation disorders |
Premature ejaculation |
Retarded or delayed ejaculation |
Anejaculation |
Painful ejaculation |
Retrograde ejaculation |
Anorgasmia |
Haemospermia |
6.2. Premature ejaculation
6.2.1. Epidemiology
Historically, the main problem in assessing the prevalence of PE has been the lack of a universally recognised definition at the time that surveys were conducted [215]. See Section 4.2 for a comprehensive discussion of the epidemiology of PE.
6.2.2. Pathophysiology and risk factors
The aetiology of PE is unknown, with few data to support suggested biological and psychological hypotheses, including anxiety [679-683], penile hypersensitivity [684-691] and 5-hydroxytryptamine (HT) receptor dysfunction [692-697]. The classification of PE into four subtypes [224] has contributed to a better delineation of lifelong, acquired, variable and subjective PE [698-700]. It has been hypothesised that the pathophysiology of lifelong PE is mediated by a complex interplay of central and peripheral serotonergic, dopaminergic, oxytocinergic, endocrinological, genetic and epigenetic factors [701]. Acquired PE may occur due to psychological problems - such as sexual performance anxiety, and psychological or relationship problems and/or co-morbidity, including ED, prostatitis, hyperthyroidism and poor sleep quality [702-705].
A significant proportion of men with ED also experience PE [232,340]. High levels of performance anxiety related to ED may worsen PE, with a risk of misdiagnosing PE instead of the underlying ED. According to the National Health and Social Life Survey (NHSLS), the prevalence of PE is not affected by age [220], unlike ED, which increases with age. Conversely, other data depicted an increased prevalence with ageing [683]; for instance, Verze et al. reported that PE prevalence based on the Premature Ejaculation Diagnostic Tool (PEDT) score (> 11) [706] proportionally increased with age [707]. Similarly, in a systematic review, PE was found to be more common in older age, with peak prevalence in men aged 60-69 years [708]. Premature ejaculation is not affected by marital or income status [220,707]. However, PE is more common in Black men, Hispanic men, and men from regions where an Islamic background is common [219,709] and prevalence may be higher in men with a lower educational level [220,232]. Other reported risk factors for PE include genetic predisposition [697,710-713], poor overall health status and obesity [220], prostate inflammation [318,714-717], hyperthyroidism [702], low prolactin levels [718], high testosterone levels [719], vitamin D and B12 deficiency [720,721], diabetes [722,723], MetS [724,725], lack of physical activity [726], emotional problems and stress [220,727,728], depressive symptoms [728], and traumatic sexual experiences [220,232]. In the only published study on risk modification/prevention strategies [729], successful eradication of causative organisms in patients with chronic prostatitis and PE produced marked improvements in intravaginal ejaculatory latency time (IELT) and ejaculatory control compared to untreated patients.
6.2.3. Impact of PE on quality of life
Men with PE are more likely to report low satisfaction with their sexual relationship, low satisfaction with sexual intercourse, difficulty relaxing during intercourse, and less-frequent intercourse [730-732]. However, the negative impact of PE extends beyond sexual dysfunction. Premature ejaculation can have a detrimental effect on self-confidence and the relationship with the partner, and may sometimes cause mental distress, anxiety, embarrassment and depression [730,733,734]. Moreover, PE may also affect the partner’s sexual functioning and their satisfaction with the sexual relationship decreases with increasing severity of the patient’s condition [735-737]. Despite the possible serious psychological and QoL consequences of PE, few men seek treatment. In the Global Study of Sexual Attitudes and Behaviors survey, 78% of men who self-reported a sexual dysfunction sought no professional help or advice for their sexual problems [232], with men more likely to seek treatment for ED than for PE [232]. In the Premature Ejaculation Prevalence and Attitudes (PEPA) survey, only 9% of men with self-reported PE consulted a physician [221]. The main reasons for not discussing PE with their physician are embarrassment and a belief that there is no treatment. Physicians are often uncomfortable discussing sexuality with their patients usually because of embarrassment and a lack of training or expertise in treating PE [738,739]. Therefore, the majority of men (51.7%) with PE do not receive pharmacotherapy for their sexual problems [740]. Physicians need to encourage their patients to talk about PE [741].
6.2.4. Classification
There is still little consensus about the definition and classification of PE [742]. It is now universally accepted that “premature ejaculation” is a broad term that includes several concepts belonging to the common category of PE. The most recent definition comes from the International Classification of Diseases 11th Revision, where PE was renamed as Early Ejaculation [743]: “Male early ejaculation is characterized by ejaculation that occurs prior to or within a very short duration of the initiation of vaginal penetration or other relevant sexual stimulation, with no or little perceived control over ejaculation. The pattern of early ejaculation has occurred episodically or persistently over a period of at least several months and is associated with clinically significant distress.”
This definition includes four categories: male early ejaculation, lifelong generalised and situational, acquired generalised and situational, and unspecified.
In the Diagnostic and Statistical Manual of Mental Disorders V (DSM-V), PE is defined as a sexual disorder with:
- consistent ejaculation within 1 minute or less of vaginal penetration;
- over a period of at least six months;
- experienced 75–100% of the time;
- the condition results in clinically significant distress, sexual frustration, dissatisfaction, or tension between partners;
- this condition is not better accounted for by another non-sexual mental disorder, medication or illicit substance use, or medical condition [239].
The EAU Guidelines have adopted the definition of PE that was developed by the International Society for Sexual Medicine (ISSM) as the first evidence-based definition [744]. According to this definition, PE (lifelong and acquired) is a male sexual dysfunction characterised by the following:
- ejaculation that always or nearly always occurs prior to or within about 1 minute of vaginal penetration (lifelong PE) or a clinically significant and bothersome reduction in latency time, often to about 3 minutes or less (acquired PE);
- inability to delay ejaculation on all or nearly all vaginal penetrations;
- negative personal consequences, such as distress, bother, frustration, and/or the avoidance of sexual intimacy.
Two more PE syndromes have been proposed [699]:
- ‘Variable PE’ is characterised by inconsistent and irregular early ejaculations, representing a normal variation in sexual performance.
- ‘Subjective PE’ is characterised by subjective perception of consistent or inconsistent rapid ejaculation during intercourse, while ejaculation latency time is in the normal range or can even last longer. It should not be regarded as a symptom or manifestation of true medical pathology.
The addition of these new syndrome types may help in overcoming the limitations of each individual definition, and it may support a more flexible view of PE for patient stratification, diagnosis and treatment [745].
6.2.5. Diagnostic evaluation
Diagnosis of PE is based on the patient’s medical and sexual history [228,746,747]. History should classify PE as lifelong or acquired and determine whether PE is situational (under specific circumstances or with a specific partner) or consistent. Special attention should be given to the duration time of ejaculation, degree of sexual stimulus, impact on sexual activity and QoL, and drug use or abuse. It is also important to distinguish PE from ED. Many patients with ED develop secondary PE caused by the anxiety associated with difficulty in attaining and maintaining an erection [340,748]. Furthermore, some patients are unaware that loss of erection after ejaculation is normal and may erroneously complain of ED, while the actual problem is PE [749]. There are several overlapping definitions of PE, with four shared factors (Table 17), resulting in a multi-dimensional diagnosis [750].
Table 17: Common factors in different definitions of PE
Common factors in different definitions of PE |
Time to ejaculation assessed by IELT |
Perceived control |
Distress, bother, frustration, interpersonal difficulty related to the ejaculatory dysfunction |
6.2.5.1. Intravaginal ejaculatory latency time (IELT)
Although it has been suggested as an objective diagnostic criterion and treatment outcome measure [751,752], the use of IELT alone is not sufficient to define PE, as there is significant overlap between men with and without PE [753,754]. Moreover, some men may experience PE in their non-coital sexual activities (e.g., during masturbation, oral sex or anal intercourse); thus, measuring IELT will not be suitable for their assessment. Although PE is apparently less prevalent and less bothersome among MSM [755], many of them may also suffer from PE and IELT cannot be applied to them [756,757]. Although some studies demonstrated that MSM report longer ejaculation latency time compared to straight men [755], some others failed to demonstrate such a difference [758].
Intravaginal ejaculatory latency time has a significant direct effect on perceived control over ejaculation, but not a significant direct effect on ejaculation-related personal distress or satisfaction with sexual intercourse [731]. In addition, perceived control over ejaculation has a significant direct effect on both ejaculation-related personal distress and satisfaction with sexual intercourse (each showing direct effects on interpersonal difficulty related to ejaculation) [759]. In everyday clinical practice, self-estimated IELT is sufficient [216]. Self-estimated and stopwatch-measured IELT are interchangeable and correctly assign PE status with 80% sensitivity and 80% specificity [760]. Specificity can be improved further to 96% by combining IELT with a single-item patient-reported outcome measure (PROM) scale on control over ejaculation and satisfaction with sexual intercourse (0 = very poor, to 4 = very good) and on personal distress and interpersonal difficulty (0 = not at all, to 4 = extremely). However, self-estimated IELT may be over-estimated by ~1 minute; therefore, it must be carefully substituted with stopwatch-measured IELT while identifying men with the complaint of lifelong PE in a clinical setting [761].
Measurement of IELT with a calibrated stopwatch is mandatory in clinical trials. For any drug treatment study of PE, Waldinger et al. suggested using geometric mean instead of arithmetic mean IELT because the distributed IELT data are skewed. Otherwise, any treatment-related ejaculation delay may be overestimated if the arithmetic mean IELT is used instead of the geometric mean IELT [762].
6.2.5.2. Premature ejaculation assessment questionnaires
The need to objectively assess PE has led to the development of several questionnaires based on using PROMs. Only two questionnaires can discriminate between patients who have PE and those who do not:
- Premature Ejaculation Diagnostic Tool (PEDT): A five-item questionnaire based on focus groups and interviews from the USA, Germany, and Spain assesses control, frequency, minimal stimulation, distress and interpersonal difficulty [763]. A total score of > 11 suggests a diagnosis of PE, 9 or 10 suggests a probable diagnosis , and < 8 indicates a low likelihood of PE.
- Arabic Index of Premature Ejaculation (AIPE): A seven-item questionnaire developed in Saudi Arabia assesses sexual desire, hard erections for sufficient intercourse, time to ejaculation, control, satisfaction of the patient and partner, and anxiety or depression [764]. A cut-off score of 30 (range 7-35) discriminates PE diagnosis best. Severity of PE is classified as severe (score: 7-13), moderate (score: 14-19), mild-to-moderate (score: 20-25) and mild (score: 26-30).
Although it is widely used, some studies have reported a low correlation between a diagnosis provided by PEDT and a self-reported diagnosis. Only 40% of men with PEDT-diagnosed PE and 19% with probable PE self-reported the condition [765]. On the contrary, a recent study has shown that PEDT is valid in screening the presence of evidence-based-defined lifelong PE and acquired PE [62]. Questionnaires are a significant step in simplifying the methodology of PE drug studies, although further cross-cultural validation is needed [760].
Other questionnaires used to characterise PE and determine treatment effects include the Premature Ejaculation Profile (PEP) [754], Index of Premature Ejaculation (IPE) [766] and Male Sexual Health Questionnaire Ejaculatory Dysfunction (MSHQ-EjD) [767]. Currently, their role is optional in everyday clinical practice. The Masturbatory Premature Ejaculation Diagnostic Tool (MPEDT) has also been recently proposed [768], due to fact that PE patients report longer IELTs and lesser bother/distress during masturbation than partnered sex [769]; however, further validation studies are required before the routine use of this questionnaire in this population.
6.2.5.3. Physical examination and investigations
Physical examination may be part of the initial assessment of men with PE. It may include a focused examination of the urological, endocrine and neurological systems to identify underlying medical conditions associated with PE or other sexual dysfunctions, such as endocrinopathy, Peyronie’s disease, urethritis or prostatitis. Laboratory or physiological testing should be directed by specific findings from history or physical examination and is not routinely recommended [746].
6.2.5.4. Recommendations for the diagnostic evaluation of PE
Recommendations | Strength rating |
Perform the diagnosis and classification of premature ejaculation (PE) based on medical and sexual history, which should include assessment of intravaginal ejaculatory latency time (IELT) (self-estimated), perceived control, distress and interpersonal difficulty due to the ejaculatory dysfunction. | Strong |
Use either stopwatch measured IELT or self-estimated IELT in clinical practice. | Weak |
Use patient-reported outcomes in daily clinical practice. | Weak |
Include physical examination in the initial assessment of PE to identify anatomical abnormalities that may be associated with PE or other sexual dysfunctions, particularly erectile dysfunction. | Strong |
Do not perform routine laboratory or neuro-physiological tests. They should only be directed by specific findings from history or physical examination. | Strong |
6.2.6. Disease management
Before commencing any treatment, it is essential to define the subtype of PE and discuss patient’s expectations thoroughly. Pharmacotherapy must be considered the first-line treatment for patients with lifelong PE, whereas treating the underlying cause (e.g., ED, prostatitis, LUTS, anxiety and hyperthyroidism) must be the initial goal for patients with acquired PE [228]. Various behavioural techniques may be beneficial in treating variable and subjective PE [770]. Psychotherapy can also be considered for PE patients who are uncomfortable with pharmacological therapy or in combination with pharmacological therapy [771,772]. However, there is weak and inconsistent evidence regarding the effectiveness of these psychosexual interventions and their long-term outcomes in PE are unknown [773].
In lifelong PE, behavioural techniques are not recommended alone, and pharmacotherapy must be considered as the basis of treatment [228]. Dapoxetine (30 and 60 mg) is the first on-demand oral pharmacological agent approved for lifelong and acquired PE in many countries, except for the USA [774]. The metered-dose aerosol spray of lidocaine (150 mg/mL) and prilocaine (50 mg/mL) combination is the first topical formulation to be officially approved for on-demand treatment of lifelong PE by the EMA in the European Union [775]. All other medications used in PE are off-label indications [766]. In this context, daily or on-demand use of selective serotonin re-uptake inhibitors (SSRIs) and clomipramine and on-demand topical anaesthetic agents have consistently shown efficacy in PE [776-779]. The long-term outcomes of pharmacological treatments are unknown. An evidence-based analysis of all current treatment modalities was performed. Levels of evidence and grades of recommendation are provided, and a treatment algorithm is presented (Figure 7).
Figure 7: Management of premature ejaculation*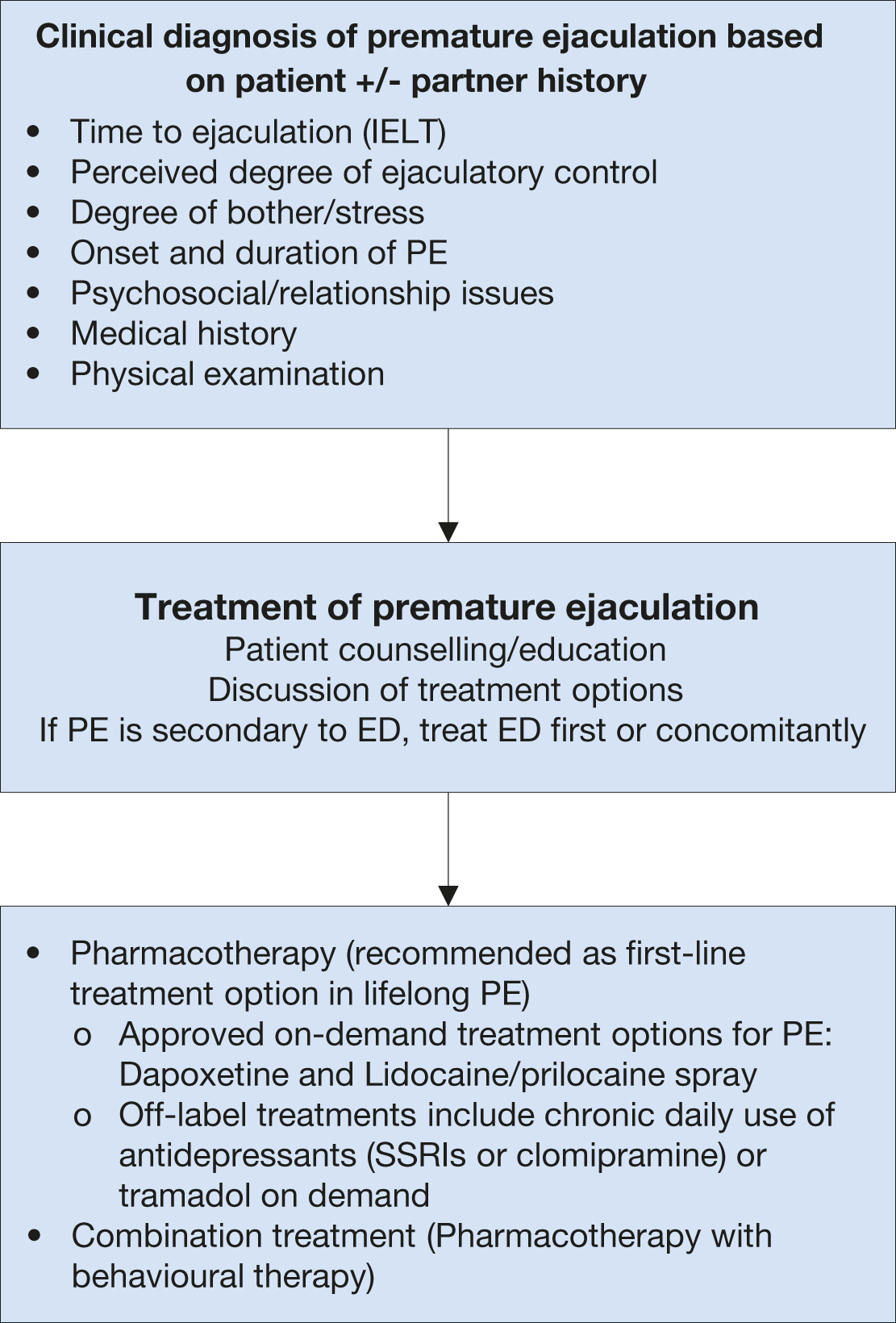
* Adapted from Lue et al. 2004 [780].
ED = erectile dysfunction; PE = premature ejaculation; IELT = intravaginal ejaculatory latency time; SSRI = selective serotonin receptor inhibitor.
6.2.6.1. Psychological aspects and intervention
Only a few studies have addressed the psychological factors underpinning PE. Men with PE have been shown to present dysfunctional responsibility attribution patterns regarding their sexual experience. These men blame themselves for their dysfunctional sexual response, even when the negative sexual outcome is unrelated to early ejaculation; additionally, they take less credit for any positive sexual experience they might have [781,782]. In addition to this style of internalised blame, men with PE focus on bodily sensations and their partners’ reactions during sex to monitor potential signs of a threat to their sexual performance. This monitoring process denotes a dysfunctional cognitive and attention style that contributes to the maintenance of PE [431]. Premature ejaculation is further related to increased levels of anxiety, including social anxiety [431,759]. Yet, it is not known whether anxiety is a precursor or a consequence of PE [680]. Men with PE reported more caution, worry, and less motivation toward novelty and exciting situations; that personality style may eventually intersect with PE dynamics [783]. The negative impact of PE on couples has been consistently mentioned. Female partners of men with PE present an increased likelihood of sexual dysfunction [784,785]; the intimate sphere, as well as the overall relationship quality, is compromised by PE [773]. An important trigger for seeking help in PE is partner dissatisfaction and the negative impact of PE on the general QoL of the couple [786]. Accordingly, psychosexual interventions, whether behavioural, cognitive, or focused on the couple, are aimed at teaching techniques to control/delay ejaculation, gaining confidence in sexual performance, reducing anxiety, and promoting communication and problem-solving within the couple [770]. Interventions with a focus on sexual education or acceptance may be positive as well [787]. It is worth noting, however, that psychosexual interventions alone regarding PE lack empirical support. Recent evidence suggests that start-stop exercises, combined with psycho-education and mindfulness techniques improve PE symptoms, as well as PE-associated distress, anxiety and depression [788]. The potential benefits of mindfulness have been reported [789]. Behavioural therapy may be most effective when used to ‘add value’ to medical interventions. In a prospective, randomised trial, combining dapoxetine and behavioural treatment was more effective than dapoxetine alone in patients with lifelong PE [771]. Smartphone-delivered psychological intervention, aimed at improving behavioural skills for ejaculatory delay and sexual self-confidence, has positive effects, supporting E-health in the context of PE [790]. Validated assessment instruments need to be used as end-points. Longer follow-up periods are necessary to confirm these findings.
Figure 8: Key aspects for psychosexual evaluation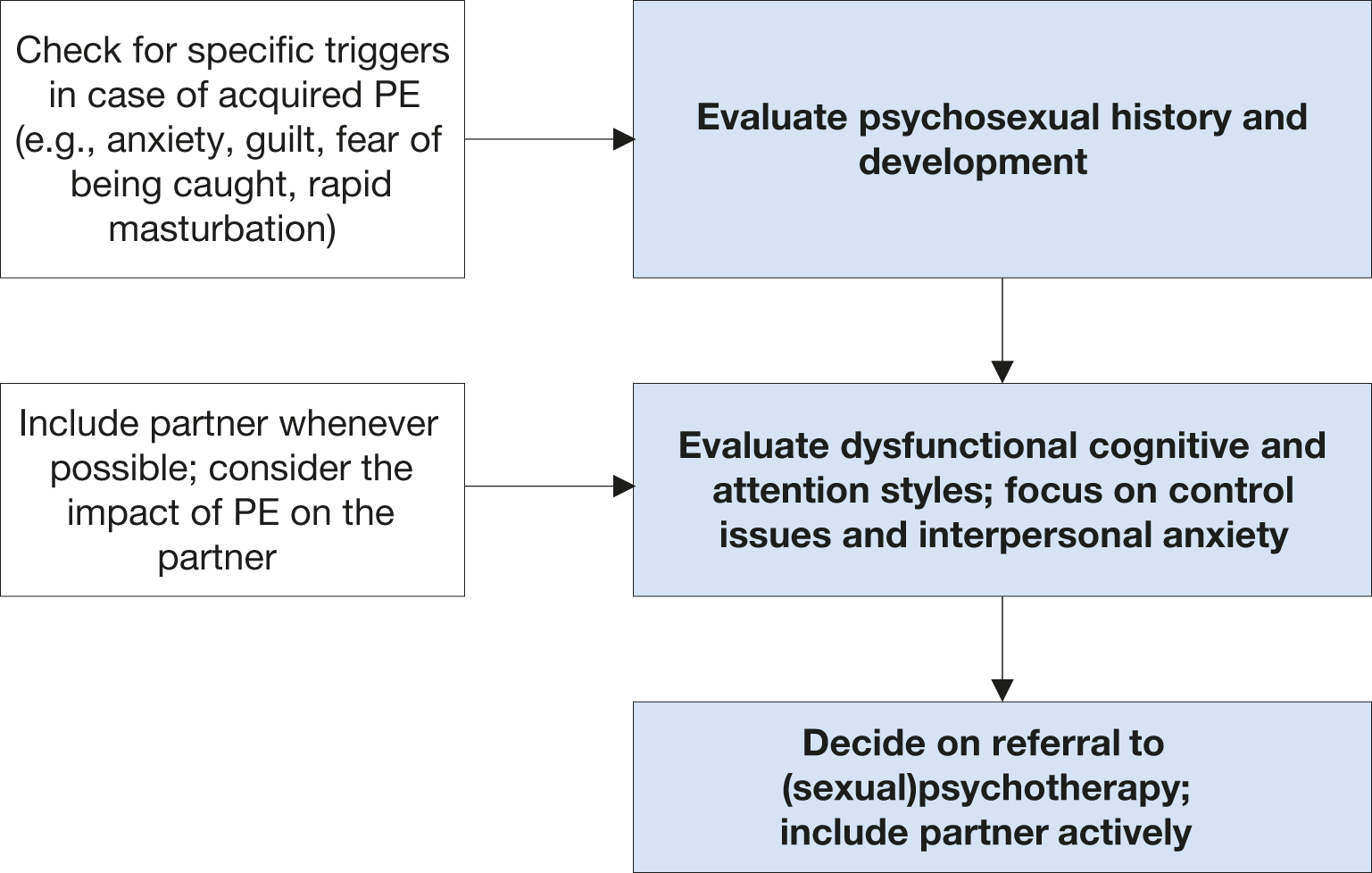
6.2.6.1.1. Recommendation for the assessment and treatment (psychosexual approach) of PE
Recommendations for assessment | Strength rating |
Consider sexual history and psychosexual development. | Strong |
Consider anxiety, and interpersonal anxiety; focus on control issues. | Strong |
Include partner if available; check for the impact of PE on the partner. | Strong |
Recommendation for treatment (psychosexual approach) | |
Use behavioural, cognitive and/or couple therapy approaches. Consider mindfulness exercises. | Weak |
6.2.6.2. Pharmacotherapy
6.2.6.2.1. Dapoxetine
Dapoxetine hydrochloride is a short-acting SSRI with a pharmacokinetic profile suitable for on-demand treatment for PE [766]. It has a rapid Tmax (1.3 hours) and a short half-life (95% clearance rate after 24 hours) [791,792]. It is approved for on-demand treatment of PE in European countries and elsewhere, but not in the USA. Both available doses of dapoxetine (30 mg and 60 mg) have shown 2.5- and 3.0-fold increases, respectively, in IELT overall, rising to 3.4- and 4.3-fold in patients with a baseline average IELT < 30 seconds [774,793-795].
In RCTs, dapoxetine, 30 mg or 60 mg 1-2 hours before intercourse, was effective at improving IELT and increasing ejaculatory control, decreasing distress, and increasing satisfaction [793]. Dapoxetine has shown a similar efficacy profile in men with lifelong and acquired PE [774,796,797]. Treatment-related adverse effects were dose-dependent and included nausea, diarrhoea, thirst, headache and dizziness [795]. Treatment-emergent adverse events (TEAEs) were responsible for study discontinuation in 4% (30 mg) and 10% (60 mg) of subjects [216]. There was no indication of an increased risk of suicidal ideation or suicide attempts and little indication of withdrawal symptoms with abrupt dapoxetine cessation [793,798]. Dapoxetine is safer than formal anti-depressant compounds used for treatment of PE [799].
A low rate (0.1%) of vasovagal syncope was reported in phase 3 studies [800]. According to the summary of product characteristics, vital orthostatic signs (blood pressure and heart rate) must be measured prior to starting dapoxetine, and dose titration must be considered [801]. The EMA assessment report for dapoxetine concluded that the potentially increased risk for syncope had been proven manageable with adequate risk minimisation measures [802]. No cases of syncope were observed in a post-marketing observational study, which identified patients at risk for the orthostatic reaction using the patient’s medical history and orthostatic testing [803].
Many patients and physicians may prefer using dapoxetine in combination with a PDE5I to extend the time until ejaculation and minimise the risk of ED due to dapoxetine treatment. Phase 1 studies of dapoxetine have confirmed that it has no pharmacokinetic interactions with PDE5Is (i.e., tadalafil 20 mg and sildenafil 100 mg) [804]. When dapoxetine is co-administered with PDE5Is, it is well tolerated, with a safety profile consistent with previous phase 3 studies of dapoxetine alone [805]. A recent RCT, including PE patients without ED, demonstrated that a combination of dapoxetine with sildenafil could significantly improve IELT values and PROMs compared with dapoxetine alone or sildenafil alone, with tolerable adverse events [806]. The efficacy and safety of dapoxetine/sildenafil combination tablets for the treatment of PE have also been reported [807].
Although dapoxetine is the only EMA-approved oral drug for treatment of PE, discontinuation rates seem moderate to high [808]. The cumulative discontinuation rates increase over time, reaching 90% at 2 years after initiation of therapy. The reasons for the high discontinuation rate are cost (29.9%), disappointment that PE was not curable and the on-demand nature of the drug (25%), adverse effects (11.6%), perceived poor efficacy (9.8%), a search for other treatment options (5.5%), and unknown (18.3%) [809]. Similarly, it was confirmed that many patients on dapoxetine treatment spontaneously discontinued treatment, while this rate was reported at 50% for other SSRIs and 28.8% for paroxetine [810]. In a Chinese cohort study, 13.6% of the patients discontinued dapoxetine due to lack of efficacy (62%), adverse effects (24%), and low frequency of sexual intercourse (14%) [811].
6.2.6.2.2. Off-label use of antidepressants: selective serotonin reuptake inhibitors and clomipramine
Ejaculation is commanded by a spinal ejaculation generator [812,813] under excitatory or inhibitory influences from the brain and the periphery [722]. 5-hydroxytryptamine (5-HT or serotonin) is involved in ejaculatory control, with its ejaculation-retarding effects likely to be attributable to the activation of 5-HT1B and 5-HT2C receptors, both spinally and supraspinally. By contrast, stimulation of 5-HT1A receptors precipitates ejaculation [814].
Selective serotonin re-uptake inhibitors are used to treat mood disorders but can delay ejaculation and therefore have been widely used ‘off-label’ for PE since the 1990s [815]. For depression, SSRIs must be given for 1-2 weeks to be effective for PE [814]. Administration of chronic SSRIs causes prolonged increases in synaptic cleft serotonin, which desensitises the 5-HT1A and 5-HT1B receptors [816]. Commonly used SSRIs include continuous intake of citalopram, fluoxetine, fluvoxamine, paroxetine and sertraline, all of which have similar efficacy, whereas paroxetine exerts the most substantial ejaculation delay [751,817,818]. Recently, a novel 5-HT1A receptor antagonist, GSK958108, significantly delayed ejaculation in a double-blind, placebo-controlled trial [819].
Clomipramine, the most serotoninergic tricyclic antidepressant, was first reported in 1977 as an effective PE treatment [820,821]. In a recent RCT, on-demand use of clomipramine 15 mg, 2-6 hours before sexual intercourse was found to be associated with IELT fold change and significant improvements in PROMs in the treatment group as compared with the placebo group (4.66 ± 5.64 vs. 2.80 ± 2.19, P < 0.05). [822,823]. The most commonly reported TEAEs were nausea in 15.7% and dizziness in 4.9% of men, respectively [822,823].
Several systematic reviews and meta-analyses of drug treatment have reported that, despite methodological problems in most studies, there remain several, well-designed, double-blind, placebo-controlled trials supporting the therapeutic effect of daily SSRIs on PE [751,776-779,824]. These meta-analyses suggest SSRIs may increase the geometric mean IELT by 2.6-13.2-fold. Paroxetine is superior to fluoxetine, clomipramine and sertraline [825,826]. Sertraline is superior to fluoxetine, whereas the efficacy of clomipramine is not significantly different from that of fluoxetine and sertraline. Paroxetine was evaluated in doses of 20-40 mg, sertraline 25-200 mg, fluoxetine 10-60 mg and clomipramine 25-50 mg. There was no significant relationship between dose and response among the various drugs. There is limited evidence that citalopram may be less efficacious compared to other SSRIs, while fluvoxamine may not be effective [827,828].
Ejaculation delay may start a few days after drug intake, but it is more evident after 1-2 weeks as receptor desensitisation requires time to occur. Although efficacy may be maintained for several years, tachyphylaxis (decreasing response to a drug following chronic administration) may occur after 6-12 months [820]. Common TEAEs of SSRIs include fatigue, drowsiness, yawning, nausea, vomiting, dry mouth, diarrhoea and perspiration; TEAEs are usually mild and gradually improve after 2-3 weeks of treatment [793,820]. Decreased libido, anorgasmia, anejaculation and ED have also been reported.
Because of the risk of suicidal ideation or suicide attempts, caution is suggested in prescribing SSRIs to young adolescents aged < 18 years with PE, and to men with PE and a comorbid depressive disorder, particularly when associated with suicidal ideation. Patients should be advised to avoid sudden cessation or rapid dose reduction of daily-dosed SSRIs, which may be related to SSRI withdrawal syndrome [216]. Moreover, PE patients trying to conceive should avoid using these medications because of their detrimental effects on sperm cells [829-832].
6.2.6.2.3. Topical anaesthetic agents
The use of local anaesthetics to delay ejaculation is the oldest form of pharmacological therapy for PE [833]. Several trials [687,834,835] support the hypothesis that topical desensitising agents reduce the sensitivity of the glans penis thereby delaying ejaculatory latency, but without adversely affecting the sensation of ejaculation. Meta-analyses have confirmed the efficacy and safety of these agents for the treatment of PE [836,837]. In a recent meta-analysis, the efficacy of local anaesthetics was best among the other treatment options including SSRIs, dapoxetine 30 and 60 mg, PDE5Is and tramadol for < 8 weeks of therapy [838].
6.2.6.2.3.1. Lidocaine/prilocaine cream
In a randomised, double-blind, placebo-controlled trial, lidocaine/prilocaine cream increased IELT from one minute in the placebo group to 6.7 minutes in the treatment group [839]. In another randomised, double-blind, placebo-controlled trial, lidocaine/prilocaine cream significantly increased the stopwatch-measured IELT from 1.49-8.45 minutes, while no difference was recorded in the placebo group (1.67-1.95 minutes) [840]. Although no significant TEAEs have been reported, topical anaesthetics are contraindicated in patients or partners with an allergy to any ingredient in the product. These anaesthetic creams/gels may be transferred to the partner, resulting in vaginal numbness. Therefore, patients are advised to use a condom after applying the cream to their penis. Alternatively, the penis can be washed to clean off any residual active compound prior to sexual intercourse. Since these chemicals may be associated with cytotoxic effects on fresh human sperm cells, couples seeking parenthood should not use topical lidocaine/prilocaine-containing substances [841].
6.2.6.2.3.2. Lidocaine/prilocaine spray
The eutectic lidocaine/prilocaine spray is a metered-dose aerosol spray containing purely base forms of lidocaine (150 mg/mL) and prilocaine (50 mg/mL), which has been officially approved by the EMA for the treatment of lifelong PE [842]. Compared to topical creams, the metered-dose spray delivery system has been proved to deposit the drug in a dose-controlled, concentrated film covering the glans penis, maximising neural blockage and minimising the onset of numbness [843], without absorption through the penile shaft skin [844].
To date, one phase 2 proof-of-concept [844], two phase 3 RCTs [845,846], and one post-marketing study [847] have demonstrated the efficacy of lidocaine/prilocaine spray in improving both IELT and the Index of Ejaculatory Control of patients with primary PE, along with an improvement in scores assessing treatment satisfaction (IPE) [845,846]. A dose/time-finding study demonstrated that the best method of intake for such sprays to optimise treatment outcomes is three sprays administered 5 min before sexual intercourse [848].
Based on these data, according to the patient information leaflet [849], the recommended dose of lidocaine/prilocaine spray is one dose (namely three sprays) to be applied on the glans penis at least 5 minutes before sexual intercourse [850]. Published data showed that lidocaine/prilocaine spray increases IELT over time up to 6.3-fold over 3 months, with a month-by-month improvement through the course of the treatment in long-term studies [851]. A low incidence of local TEAEs in both patients and partners has been reported, including genital hypoaesthesia (4.5% and 1.0% in men and female partners, respectively) and ED (4.4%), and vulvovaginal burning sensation (3.9%), but is unlikely to be associated with systemic TEAEs [849,852].
Lidocaine-only sprays are also effective in the treatment of PE. In a recent RCT, PE patients were randomly allocated to receive either dapoxetine 60 mg or topical lidocaine 10% spray. The geometric mean IELTs were significantly better in the lidocaine compared with the dapoxetine group (179.43 vs. 63.44, respectively). However, both groups showed significant improvement compared with baseline IELTs value (63.44 and 179.4 vs. 21.87, p < 0.05) [853]. In another RCT, lidocaine 5% spray was compared with alcohol spray (placebo) in the treatment of lifelong PE for 8 weeks; the mean values of the AIPE scores, IELT, and sexual intercourse frequency in the lidocaine 5% spray group were significantly increased compared with the placebo group [854].
6.2.6.2.4. Tramadol
Tramadol is a centrally-acting analgesic agent that combines opioid receptor activation and serotonin and noradrenaline re-uptake inhibition. Tramadol is a mild-opioid receptor agonist, but it also displays antagonistic properties on transporters of noradrenaline and 5-HT [855]. This mechanism of action distinguishes tramadol from other opioids, including morphine. Tramadol is readily absorbed after oral administration and has an elimination half-life of 5-7 hours.
A large, randomised, double-blind, placebo-controlled, multicentre 12-week study was carried out to evaluate the efficacy and safety of two doses of tramadol (62 and 89 mg) by ODT in the treatment of PE [856].
A bioequivalence study has demonstrated equivalence between tramadol ODT and tramadol HCI. In patients with a history of lifelong PE and an IELT < 2 minutes, increases in the median IELT of 0.6 minutes (1.6-fold), 1.2 minutes (2.4-fold) and 1.5 minutes (2.5-fold) were reported for placebo, 62 mg of tramadol ODT, and 89 mg of tramadol ODT, respectively. It should be noted that there was no dose-response effect with tramadol. There are four RCTs comparing the efficacy of tramadol to paroxetine in the literature. On demand tramadol treatment yielded significantly higher IELTs compared to on-demand paroxetine treatment arm in three of the RCTs [857-859], whilst in the remaining RCT, daily paroxetine was found more effective to treat lifelong PE when compared with on-demand tramadol treatment [860]. Recent meta-analyses revealed that 50 mg tramadol had a significant improvement in the IELT compared with 20 mg paroxetine [861,862]. Adverse effects were reported at doses used for analgesic purposes (< 400 mg daily) and included constipation, sedation and dry mouth. In May 2009, the FDA released a warning letter about tramadol’s potential to cause addiction and difficulty in breathing [863]. The tolerability during the 12-week study period in men with PE was acceptable [858]. Several other studies have also reported that tramadol exhibits a significant dose-related efficacy along with potential adverse effects during the treatment of PE [864]. The efficacy and safety of tramadol have been confirmed in systematic reviews and meta-analyses [858,861,862,865-867]. The Guidelines Panel considers tramadol as a potential alternative treatment to established first-line therapeutic options in men with PE; however, it should be clearly outlined that the use of tramadol has to be considered with caution since there is a lack of data on the long-term safety of the compound in this setting.
6.2.6.2.5. Phosphodiesterase type 5 inhibitors
One well-designed, randomised, double-blind, placebo-controlled study compared sildenafil to placebo in men with PE [868]. Although IELT was not significantly improved, sildenafil increased confidence, the perception of ejaculatory control and overall sexual satisfaction, reduced anxiety and the refractory time to achieve a second erection after ejaculation. Other RCTs demonstrated that once-daily 5 mg tadalafil was well tolerated by patients with PE and it was effective in improving IELTs and PROMs [869,870]. However, a recently conducted single-blind placebo-controlled study failed to demonstrate statistically significant improvement in IELT and PEDT in 55 lifelong PE patients [871]. Several open-label studies have shown that a combination of PDE5Is and SSRIs is superior to SSRI monotherapy, which has also been recently confirmed by a Bayesian network meta-analysis [838]:
- Sildenafil combined with paroxetine improved IELT significantly and satisfaction vs. paroxetine alone [872];
- Sildenafil combined with sertraline improved IELT and satisfaction significantly vs. sertraline alone [873];
- Sildenafil combined with paroxetine and psychological and behavioural counselling significantly improved IELT and satisfaction in patients in whom other treatments failed [874];
- Sildenafil combined with dapoxetine (30 mg) improved IELT, satisfaction scores and PEDT vs. dapoxetine, paroxetine or sildenafil monotherapy [806,807];
- Tadalafil 5 mg once-daily treatment was effective in improving IELTs and PROMs [869,870].
- Tadalafil combined with paroxetine or dapoxetine significantly improved IELT and satisfaction vs. paroxetine, dapoxetine or tadalafil alone [875,876];
- Sildenafil combined with behavioural therapy significantly improved IELT and satisfaction vs. behavioural therapy alone [877].
Overall, there are limited data on the efficacy of other PDE5Is (tadalafil and vardenafil) [878,879]. In a recent meta-analysis, PDE5Is were found to be significantly more effective than placebo in the treatment of patients with PE and without ED [880]. Some meta-analyses have demonstrated that the combined use of SSRIs and PDE5Is may be more effective than SSRI or PDE5I monotherapy [778,881-885]. In a recent Bayesian meta-analysis, combined therapy of SSRI and PDE5I was found to be superior to other treatment modalities (including topical creams, tramadol, paroxetine or fluoxetine monotherapy, PDE5I monotherapy, dapoxetine
30 and 60 mg, clomipramine, citalopram, and placebo) lasting > 8 weeks [838].
6.2.6.2.6. Other drugs
In addition to the aforementioned drugs, there is continuous research into other treatment options. Considering the abundant α1a-adrenergic receptors in seminal vesicles and the prostate and the role of the sympathetic system in ejaculation physiology, the efficacy of selective α-blockers in the treatment of PE has been assessed [886-888]. A recent study demonstrated that wake-promoting agent modafinil may be effective in delaying ejaculation and improving PROMs [889].
Decreasing penile sensitivity with glans penis augmentation using hyaluronic acid for the treatment of PE was initially proposed by Korean researchers in 2004 [890]. Since then, it has gained popularity mainly in Asian countries [891-896]. In a randomised controlled cross-over study, hyaluronic acid glans injections were safe, with a modest but significant increase in IELT [897]. A more recent prospective, patient/evaluator-blinded, randomised, active-controlled, multicentre trial, showed significant improvement in PEP index scores and self-estimated IELT (from 5.36 ± 3.51 to 7.86 ± 4.73 minutes, p = 0.0001) [895]. In RCTs, no serious TEAEs were reported related to glans penis injections with hyaluronic acid. Improvement in IELT by hyaluronic acid injections in persistent PE patients has also been demonstrated in a prospective controlled study [893].
Selective dorsal neurectomy has also been suggested for the treatment of PE by Asian researchers [898-904]. However, this procedure may result in serious complications, and more safety studies must be conducted before recommending this treatment to PE patients [905].
Considering the importance of central oxytocin receptors in the ejaculation reflex, several researchers have assessed the efficacy and safety of oxytocin receptor antagonists in the treatment of PE [906]. Epelsiban [907] and cligosiban [908-910] have been found to be safe and mildly effective in delaying ejaculation, but further controlled trials are needed [910].
Retarded ejaculation was associated with the use of pregabalin, a new generation of gapapentinoid, as a side-effect. In a double-blind, placebo-controlled randomised trial where the efficacy and tolerability of on-demand oral pregabalin (150 mg and 75 mg) in the treatment of PE was trialled, it was found that IELTs of patients who received 150 mg pregabalin improved significantly (2.45 ± 1.43-fold) compared to those who received 75 mg pregabalin and placebo. Treatment-emergent side effects (blurred vision, dizziness, vomiting) were minimal and did not lead to drug discontinuation.
The role of other proposed treatment modalities for the treatment of PE, such as penis-root masturbation [911], vibrator-assisted start-stop exercises [788], transcutaneous functional electric stimulation [912], transcutaneous posterior tibial nerve stimulation [913], acupuncture [914-916] and practicing yoga [917] need more evidence to be considered in the clinical setting.
6.2.7. Summary of evidence on the epidemiology/aetiology/pathophysiology of PE
Summary of evidence | LE |
Pharmacotherapy includes either dapoxetine on-demand (an oral short-acting SSRI) and eutectic lidocaine/prilocaine spray (a topical desensitising agent), which are the only approved treatments for PE, or other off-label antidepressants (daily/on-demand SSRIs and clomipramine). | 1a |
6.2.8. Recommendations for the treatment of PE
Recommendations | Strength rating |
Treat erectile dysfunction (ED), other sexual dysfunction or genitourinary infection (e.g., prostatitis) first. | Strong |
Use either dapoxetine or the lidocaine/prilocaine spray as first-line treatments for lifelong premature ejaculation (PE). | Strong |
Use off-label topical anaesthetic agents as a viable alternative to oral treatment with selective serotonin re-uptake inhibitor (SSRIs). | Strong |
Use off-label tramadol with caution as a viable on-demand alternative to on-demand SSRIs. | Strong |
Use PDE5Is alone or in combination with other therapies in patients with PE (without ED). | Strong |
Use psychological/behavioural therapies in combination with pharmacological treatment in the management of acquired PE. | Weak |
Use hyaluronic acid injection with caution as a treatment option for PE compared to other more established treatment modalities. | Weak |
6.3. Delayed Ejaculation
6.3.1. Definition and classification
The American Psychiatric Association defines DE as requiring one of two symptoms: marked delay, infrequency or absence of ejaculationon 75-100% of occasions that persists for at least 6 months and causes personal distress [239]. However, in a recent study, while ejaculatory latency and control were significant criteria to differentiate men with DE from those without ejaculatory disorders, bother/distress did not emerge as a significant factor [918]. Similar to PE, there are distinctions among lifelong, acquired and situational DE [239]. Although the evidence is limited, the prevalence of lifelong and acquired DE is estimated at around 1% and 4%, respectively [240].
6.3.2. Pathophysiology and risk factors
The aetiology of DE can be psychological, organic (e.g., incomplete spinal cord lesion or iatrogenic penile nerve damage), or pharmacological (e.g., SSRIs, antihypertensive drugs, or antipsychotics) [919,920] (Table 18). Other factors that may play a role in the aetiology of DE include tactile sensitivity and tissue atrophy [787]. Although low testosterone level has been considered a risk factor in the past [57,719], more contemporary studies have not confirmed any association between ejaculation times and serum testosterone levels [921,922]. Idiosyncratic masturbation and lack of desire for stimuli are also proposed risk factors for DE [234-236].
Table 18: Aetiological causes of delayed ejaculation and anejaculation
Ageing Men | Degeneration of penile afferent nerves inhibited ejaculation |
Congenital | Mullerian duct cyst Wolfian duct abnormalities Prune Belly Syndrome Imperforate Anus Genetic abnormalities |
Anatomic causes | Transurethral resection of prostate Bladder neck incision Circumcision Ejaculatory duct obstruction (can be congenital or acquired) |
Neurogenic causes | Diabetic autonomic neuropathy Multiple sclerosis Spinal cord injury Radical prostatectomy Proctocolectomy Bilateral sympathectomy Abdominal aortic aneurysmectomy Para-aortic lymphadenectomy |
Infective/Inflammation | Urethritis Genitourinary tuberculosis Schistosomiasis Prostatitis Orchitis |
Endocrine | Hypogonadism Hypothyroidism Prolactin disorders |
Medication | Antihypertensives; thiazide diuretics Alpha-adrenergic blockers Antipsychotics and antidepressants Alcohol Antiandrogens Ganglion blockers Selective serotonin reuptake Inhibitors |
Psychological | Acute psychological distress Relationship distress Psychosexual skill deficit Disconnect between arousal and sexual situations Masturbation style |
6.3.3. Investigation and treatment
Patients should have a full medical and sexual history performed along with a detailed physical examination when evaluating for DE. It is not uncommon for clinicians to feel uncomfortable with the level of sexual information that is warranted in obtaining a full sexual history. Understanding the details of the ejaculatory response, sensation, frequency, and sexual activity/techniques; cultural context and history of the disorder; quality of the sexual response cycle (desire, arousal, ejaculation, orgasm, and refractory period); partner’s assessment of the disorder and if the partner suffers from any sexual dysfunction her/himself; and the overall satisfaction of the sexual relationship are all important to garner during history-taking [924]. Investigation by a sex therapist is often required to help obtain a complete psychological evaluation. It is incumbent on the clinician to diagnose medical pathologies that cause or contribute to DE, such as assessing the hormonal milieu, anatomy, and overall medical condition. Good communication between the sex therapist and medical practitioner is vital to successfully diagnosing and treating DE.
6.3.3.1. Psychological aspects and intervention
There is scarce literature on the psychological aspects relating to DE, as well as on empirical evidence regarding psychological treatment efficacy. Studies on psychological aspects have revealed that men with DE show a strong need to control their sexual experiences. Delayed ejaculation is associated with difficulties surrendering to sexual pleasure during sex - i.e., the sense of letting go [925] - which denotes a underlying psychological mechanism influencing the reaching of orgasm [926]. As for psychological treatments, these may include, but are not limited to: increased genital-specific stimulation; sexual education; role-playing on his own and in front of his partner; retraining masturbatory practices; anxiety reduction on ejaculation and performance; and, re-calibrating the mismatch of sexual fantasies with arousal (such as with pornography use and fantasy stimulation compared to reality) [924]. A basic understanding of the sexual cycle for their respective partners can assist men and women in managing expectations and in evaluating their own sexual practices. Masturbation techniques that are either solo or partnered can be considered practice for the “real performance”, which can eventually result in greater psychosexual arousal and orgasm for both parties [236]. Although masturbation with fantasy can be harmful when not associated with appropriate sexual arousal and context, fantasy can be supportive if it allows blockage of critical thoughts that may prevent orgasm and ejaculation. Techniques geared towards reducing of anxiety are important skills that can help overcome performance anxiety, as this can often interrupt the natural erectile function through orgasmic progression. Referral to a sexual therapist, psychologist or psychiatrist is appropriate and often warranted.
6.3.3.2. Pharmacotherapy
Several pharmacological agents, including cabergoline, bupropion, alpha-1-adrenergic agonists (pseudoephedrine, midodrine, imipramine and ephedrine), buspirone, oxytocin, testosterone, bethanechol, yohimbine, amantadine, cyproheptadine and apomorphine have been used to treat DE with varied success [787]. Unfortunately, there is no FDA or EMA-approved medications to treat DE, as most of the cited research is based on case-cohort studies that were not randomised, blinded, or placebo-controlled. Many drugs have been used as primary treatments and/or antidotes to other medications that can cause DE. A recent survey of sexual health providers demonstrated an overall treatment success of 40% with most providers commonly using cabergoline, bupropion or oxytocin [927]. However, this survey measured the anecdotal results of practitioners. There was no proven efficacy or superiority of any drug due to a lack of placebo-controlled, randomised, blinded, comparative trials [923]. In addition to pharmacotherapy, penile vibratory stimulation (PVS) is also used as an adjunct therapy for DE [928]. Another study that used combined therapy of midodrine and PVS to increase autonomic stimulation in 158 men with spinal cord injury led to ejaculation in almost 65% of the patients [929].
6.4. Anejaculation
6.4.1. Definition and classification
Anejaculation involves the complete absence of antegrade or retrograde ejaculation. It is caused by the failure of semen emission from the seminal vesicles, prostate, and ejaculatory ducts into the urethra [930]. True anejaculation is usually associated with a normal orgasmic sensation and is always associated with central or peripheral nervous system dysfunction or with drugs [931].
6.4.2. Pathophysiology and risk factors
Generally, anejaculation shares similar aetiological factors with DE and retrograde ejaculation (Table 18).
6.4.3. Investigation and treatment
Drug treatment for anejaculation caused by lymphadenectomy and neuropathy, or psychosexual therapy for anorgasmia, is not effective. In all these cases, and in men who have a spinal cord injury, PVS (i.e., application of a vibrator to the penis) is the first-line therapy. In anejaculation, PVS evokes the ejaculation reflex [932], which requires an intact lumbosacral spinal cord segment. If the quality of semen is poor or ejaculation is retrograde, the couple may enter an in vitro fertilisation program whenever fathering is desired. If PVS has failed, electro-ejaculation can be the therapy of choice [933]. Other sperm-retrieval techniques may be used when electro-ejaculation fails or cannot be carried out [934]. Anejaculation following either retroperitoneal surgery for testicular cancer or total mesorectal excision can be prevented using unilateral lymphadenectomy or autonomic nerve preservation [935], respectively.
6.5. Painful Ejaculation
6.5.1. Definition and classification
Painful ejaculation is a condition in which a patient feels mild discomfort to severe pain during or after ejaculation. The pain can involve the penis, scrotum, and perineum [936].
6.5.2. Pathophysiology and risk factors
Many medical conditions can result in painful ejaculations, but it can also be an idiopathic problem. Initial reports demonstrated possible associations of painful ejaculation with calculi in the seminal vesicles [937], sexual neurasthenia [938], sexually transmitted diseases [936,939], inflammation of the prostate [260,940], PCa [941,942], BPH [258], prostate surgery [943,944], pelvic radiation [945], herniorrhaphy [946] and antidepressants [947-949]. Further case reports have suggested that mercury toxicity or Ciguatera toxin fish poisoning may also result in painful ejaculation [950,951]. Psychological issues may also be the cause of painful ejaculation, especially if the patient does not experience this problem during masturbation [952].
6.5.3. Investigation and treatment
Treating painful ejaculation must be tailored to the underlying cause if detected. Psychotherapy or relationship counselling, withdrawal of suspected agents (drugs, toxins, or radiation) [947,948,953] or the prescription of appropriate medical treatment (antibiotics, α-blockers or anti-inflammatory agents) may ameliorate painful ejaculation. Behavioural therapy, muscle relaxants, antidepressant treatment, anticonvulsant drugs and/or opioids, and pelvic floor exercises, may be implemented if no underlying cause can be identified [954,955].
6.5.3.1. Surgical intervention
If medical treatments fail, surgical operations such as TURP, transurethral resection of the ejaculatory duct and neurolysis of the pudendal nerve have been suggested [956,957]. However, there is no strong supporting evidence that surgical therapy improves painful ejaculation: therefore it must be used with caution.
6.6. Retrograde ejaculation
6.6.1. Definition and classification
Retrograde ejaculation is the total, or sometimes partial, absence of antegrade ejaculation, due to semen passing backwards through the bladder neck into the bladder. Patients may experience a normal or decreased orgasmic sensation. The causes of retrograde ejaculation can be divided into neurogenic, pharmacological, urethral, or bladder neck incompetence [936].
6.6.2. Pathophysiology and risk factors
The process of ejaculation requires complex co-ordination and interplay between the epididymis, vas deferens, prostate, seminal vesicles, bladder neck and bulbourethral glands [958]. Upon ejaculation, sperm are rapidly conveyed along the vas deferens and into the urethra via the ejaculatory ducts. From there, the semen progresses in an antegrade fashion, partly maintained by coaptation of the bladder neck and rhythmic contractions of the periurethral muscles, co-ordinated by a centrally mediated reflex [958]. Closure of the bladder neck and seminal emission is initiated via the sympathetic nervous system from the lumbar sympathetic ganglia and subsequently hypogastric nerve. Prostatic and seminal vesicle secretion, as well as contraction of the bulbo-cavernosal, ischio-cavernosal and pelvic floor muscles are initiated by the S 2-4 parasympathetic nervous system via the pelvic nerve [958].
Any factor that disrupts this reflex and inhibits contraction of the bladder neck (internal vesical sphincter) may lead to retrograde passage of semen into the bladder. These can be broadly categorised as pharmacological, neurogenic, anatomic and endocrinal causes of retrograde ejaculation (Table 19).
Table 19: Aetiology of retrograde ejaculation
Aetiology of retrograde ejaculation | |
Neurogenic | Spinal cord injury Cauda equina lesions Multiple sclerosis Autonomic neuropathy Retroperitoneal lymphadenectomy Sympathectomy or aortoiliac surgery Prostate, colorectal and anal surgery Parkinson´s disease Diabetes mellitus Psychological/behavioural |
Urethral | Ectopic ureterocele Urethral stricture Urethral valves or verumontaneum hyperplasia Congenital dopamine β-hydroxylase deficiency |
Pharmacological | Antihypertensives, thiazide diuretics α-1-Adrenoceptor antagonists Antipsychotics and antidepressants |
Endocrine | Hypothyroidism Hypogonadism Hyperprolactinaemia |
Bladder neck incompetence | Congenital defects/dysfunction of hemitrigone Bladder neck resection (transurethral resection of the prostate) Prostatectomy |
6.6.3. Disease management
Established medical and surgical strategies now exist for the treatment of retrograde ejaculation. In recent years the reliance on medical treatment as first-line management has become more common practice.
6.6.3.1. Pharmacological
Sympathomimetics stimulate the release of noradrenaline and activate α- and β-adrenergic receptors, resulting in closure of the internal urethral sphincter, restoring the antegrade flow of semen. The most common sympathomimetics are synephrine, pseudoephedrine hydrochloride, ephedrine, phenylpropanolamine and midodrine [959]. Unfortunately, as time progresses, their effect diminishes [960]. Many studies published about the efficacy of sympathomimetics in the treatment of retrograde ejaculation suffer from small sample size, with some represented by case reports.
A double-blind controlled study randomised patients to receive one of four α-adrenergic agents (dextroamphetamine, ephedrine, phenylpropanolamine and pseudoephedrine) with or without histamine. The patients suffered from failure of ejaculation following retroperitoneal lymphadenectomy. They found that 4 days of treatment prior to ejaculation was the most effective and that all the adrenergic agonists restored antegrade ejaculation [959]. In a systematic review, the efficacy of this group of medications was found to be 28% [243]. The adverse effects of sympathomimetics include dryness of mucous membranes and hypertension.
The use of antimuscarinics has been described, including brompheniramine maleate and imipramine, as well as in combination with sympathomimetics. The calculated efficacy of antimuscarinics alone or in combination with sympathomimetics is 22% and 39%, respectively [243]. Combination therapy appears to be more effective, although statistical analysis is not yet possible due to the small sample sizes.
6.6.3.2. Management of infertility
Infertility has been the major concern of patients with retrograde ejaculation. Beyond standard sperm-retrieval techniques, such as testicular sperm extraction (TESE), three different methods of sperm acquisition have been identified for managing infertility in patients with retrograde ejaculation. These include: i) centrifugation and resuspension of post-ejaculatory urine specimens; ii) the Hotchkiss (or modified Hotchkiss) technique; and, iii) ejaculation on a full bladder.
1. Centrifugation and resuspension. In order to improve the ambient conditions for the sperm, the patient is asked to increase their fluid intake or take sodium bicarbonate to dilute or alkalise the urine, respectively. Afterwards, a post-orgasmic urine sample is collected by introducing a catheter or spontaneous voiding. This sample is then centrifuged and suspended in a medium. The types of suspension fluids are heterogeneous and can include bovine serum albumin, human serum albumin, Earle’s/Hank’s balanced salt solution and the patient’s urine. The resultant modified sperm mixture can then be used in assisted reproductive techniques. A systematic review of studies in couples in which male partner had retrograde ejaculation found a 15% pregnancy rate per cycle (0-100%) [243].
2. Hotchkiss method. The Hotchkiss method involves emptying the bladder prior to ejaculation, using a catheter, and then washing out and instilling a small quantity of Lactated Ringers to improve the ambient condition of the bladder. The patient then ejaculates, and semen is retrieved by catheterisation or voiding [961]. Modified Hotchkiss methods involve variance in the instillation medium. Pregnancy rates were 24% per cycle (0-100%) [243].
3. Ejaculation on a full bladder. The patient is encouraged to ejaculate on a full bladder and semen is suspended in Baker’s Buffer. The pregnancy rate in the two studies, which included only five patients. Few papers have described results using this technique [962,963].
6.7. Anorgasmia
6.7.1. Definition and classification
Anorgasmia is the perceived absence of orgasm and can give rise to anejaculation. Regardless of the presence of ejaculation, anorgasmia can be a lifelong (primary) or acquired (secondary) disorder [240].
6.7.2. Pathophysiology and risk factors
Primary anorgasmia starts from a man’s first sexual intercourse and lasts throughout his life, while secondary anorgasmia patients should have a normal period before the problem starts [964]. Substance abuse, obesity and some non-specific psychological aspects, such as anxiety and fear, are considered risk factors for anorgasmia. Only a few studies have described anorgasmia alone and generally, it has been considered a symptom linked to ejaculatory disorders, especially with DE, and therefore, they are believed to share the same risk factors. However, psychological factors are considered to be responsible for 90% of anorgasmia problems [965]. The causes of delayed orgasm and anorgasmia are shown in Table 20 [964].
Table 20: Causes of delayed orgasm and anorgasmia
Causes of delayed orgasm and anorgasmia | |
Endocrine | Testosterone deficiency Hypothyroidism |
Medications | Antidepressants Antipsychotics Opioids |
Psychosexual causes | |
Hyperstimulation | |
Penile sensation loss | |
6.7.3. Disease management
The psychological/behavioural strategies for anorgasmia are similar to those for DE. The patient and his partner should be examined physically and psychosexually in detail, including determining the onset of anorgasmia, medication and disease history, penile sensitivity and psychological issues. Adjunctive laboratory tests can also be used to rule out organic causes, such as testosterone, prolactin and TSH levels. Patients who have loss of penile sensitivity require further investigations [964].
6.7.3.1. Psychological/behavioural strategies
Lifestyle changes can be recommended to affected individuals, including changing masturbation style, taking steps to improve intimacy, and decreasing alcohol consumption. Several psychotherapy techniques or their combinations have been offered, including alterations in arousal methods, reduction of sexual anxiety, role-playing an exaggerated orgasm and increased genital stimulation [926,966]. However, it is difficult to determine the success rates from the literature.
6.7.3.2. Pharmacotherapy
Several drugs have been reported to reverse anorgasmia, including cyproheptadine, yohimbine, buspirone, amantadine and oxytocin [967-972]. However, these reports are generally from case-cohort studies and drugs have limited efficacy and significant adverse effect profiles. Therefore, current evidence is not strong enough to recommend drugs to treat anorgasmia.
6.7.3.3. Management of infertility
If patients fail the treatment methods mentioned above, penile vibratory stimulation, electro-ejaculation or TESE are options for sperm retrieval in anorgasmia cases [964].
6.8. Haemospermia
6.8.1. Definition and classification
Haemospermia is defined as the appearance of blood in the ejaculate. Although it is often regarded as a symptom of minor significance, blood in the ejaculate causes anxiety in many men and may indicate underlying pathology [263].
6.8.2. Pathophysiology and risk factors
Several causes of haemospermia have been acknowledged and can be classified into the following sub-categories; idiopathic, congenital malformations, inflammatory conditions, obstruction, malignancies, vascular abnormalities, iatrogenic/trauma and systemic causes (Table 21) [973].
Table 21: Pathology associated with haemospermia
Category | Causes |
Congenital | Seminal vesicle (SV) or ejaculatory duct cysts |
Inflammatory | Urethritis, prostatitis, epididymitis, tuberculosis, CMV, HIV, Schistosomiasis, hydatid, condyloma of urethra and meatus, urinary tract infections |
Obstruction | Prostatic, SV and ejaculatory duct calculi, post-inflammatory, seminal vesicle diverticula/cyst, urethral stricture, utricle cyst, BPH |
Tumours | Prostate, bladder, SV, urethra, testis, epididymis, melanoma |
Vascular | Prostatic varices, prostatic telangiectasia, haemangioma, posterior urethral veins, excessive sex or masturbation |
Trauma/iatrogenic | Perineum, testis, instrumentation, post-haemorrhoid injection, prostate biopsy, vaso-venous fistula |
Systemic | Hypertension, haemophilia, purpura, scurvy, bleeding disorders, chronic liver disease, renovascular disease, leukaemia, lymphoma, cirrhosis, amyloidosis |
Idiopathic | - |
The risk of any malignancy in patients presenting with haemospermia is approximately 3.5% (0-13.1%) [974]. In an observational study of 300 consecutive patients over a 30-month period, 81% had no identified cause of haemospermia. In those patients for whom a cause was identified, the diagnosis varied dependent upon the age of presentation. When the patients were divided into those under and those over 40 years of age, UTIs were more common among younger compared to older patients (15% vs. 10.3%). In the older group
(> 40 years), stones (2.2% vs. 1.4%) and malignancy (6.2% vs. 1.4%) were more common when compared with the younger cohort. In the > 40 years group, 13 patients had PCa and one had low-grade urethral carcinoma. In the < 40 years group, one patient had testicular cancer [262]. In a recent study in which 342 patients with haemospermia were included, the most relevant aetiology for haemospermia was inflammation/infection (49.4%) while genitourinary cancers (i.e., prostate and testis) only accounted for 3.2% of the cases [975].
6.8.3. Investigations
As with other clinical conditions, a systematic clinical history and assessment is undertaken to help identify the cause of haemospermia. Although the differential diagnosis is extensive, most cases are caused by infections or other inflammatory processes [263].
The basic examination of haemospermia should start with a thorough symptom-specific and systemic clinical history. The first step is to understand if the patient has true haemospermia. Pseudo-haemospermia may occur as a consequence of haematuria or even suction of a partner’s blood into the urethra during copulation [936,976,977]. A sexual history should be taken to identify those whose haemospermia may be a consequence of a sexually transmitted disease. Recent foreign travel to areas affected by schistosomiasis or tuberculosis should also be considered. The possibility of co-existing systemic diseases such as hypertension, liver disease and coagulopathy should be investigated along with systemic features of malignancy such as weight loss, loss of appetite or bone pain. Examination of the patient should also include measurement of blood pressure, as there have been several case reports suggesting an association between uncontrolled hypertension and haemospermia [978,979].
Most authors who propose an investigative baseline agree on the initial diagnostic tests, but there is no consensus in this regard [973,974,976,980]. Urinalysis should be performed along with sending the urine for culture and sensitivity testing, as well as microscopy. If tuberculosis or schistosomiasis is the suspected cause, the semen or prostatic secretions should be sent for analysis. A full sexually-transmitted disease screen, including first-void urine as well as serum and genitourinary samples, should be tested for Chlamydia, Ureaplasma and Herpes Simplex virus. Using this strategy, it may be possible to find an infectious agent among cases that would have been labelled as idiopathic haemospermia [981].
Serum PSA should be taken in men aged > 40 years who have been appropriately counselled [264]. Blood work, including a full blood count, liver function tests, and a clotting screen should be taken to identify systemic diseases. The question of whether further investigation is warranted depends on clinician judgment, patient age and an assessment of risk factors [973]. Digital rectal examination should also be performed, and the meatus re-examined after DRE for bloody discharge [982]. Detection of a palpable nodule in the prostate is important because an association between haemospermia and PCa has been postulated, although not completely proven.
Magnetic resonance imaging is being increasingly used as a definitive means to investigate haemospermia. The multiplanar ability of MRI to accurately represent structural changes in the prostate, seminal vesicles, ampulla of vas deferens, and ejaculatory ducts has enabled the technique to be particularly useful in determining the origin of midline or paramedian prostatic cysts and in determining optimal surgical management [983]. The addition of an endorectal coil can improve diagnostic accuracy for identifying the site and possible causes of haemorrhage [984].
Cystoscopy has been included in most suggested investigative protocols in patients with high-risk features (patients who are refractory to conservative treatment and who have persistent haemospermia). It can provide invaluable information as it allows direct visualisation of the main structures in the urinary tract that can be attributed to causes of haemospermia, such as polyps, urethritis, prostatic cysts, foreign bodies, calcifications and vascular abnormalities [985,986].
With the advancement of optics, the ability to create ureteroscopes of diameters small enough to allow insertion into the ejaculatory duct and seminal vesicles has been made possible [987]. In a prospective study, 106 patients with prolonged haemospermia underwent transrectal US and seminal vesiculoscopy. With both methods combined, the diagnosis was made in 87.7% of patients. When compared head-to-head, the diagnostic yield for TRUS vs. seminal vesiculoscopy was 45.3% and 74.5%, respectively (P < 0.001) [988].
Melanospermia is a consequence of malignant melanoma involving the genitourinary tract and is a rare condition that has been described in two case reports [989,990]. Chromatography of the semen sample can be used to distinguish the two by identifying the presence of melanin if needed.
6.8.4. Disease management
Conservative management is generally the primary treatment option when the patients are aged < 40 years and have a single episode of haemospermia. The primary goal of treatment is to exclude malignant conditions like prostate and bladder cancer and treat any other underlying cause. If no pathology is found, then the patient can be reassured [263,973].
Middle-aged patients with recurrent haemospermia warrant more aggressive intervention. Appropriate antibiotic therapy should be given to patients who have urogenital infections or STIs. Urethral or prostate varices or angiodyplastic vessels can be fulgurated, whereas cysts, either of the seminal vesicles or prostatic urethra, can be aspirated transrectally [263]. Ejaculatory duct obstruction is managed by transurethral incision at the duct opening [991,992]. Systemic conditions should be treated appropriately [974,977,993,994].
Defining a management algorithm for haemospermia is based on the patient age and degree of haemospermia. Patients often find blood in the ejaculate alarming, and investigations should be aimed at excluding a serious, despite infrequent, underlying cause (e.g., cancer), while at the same time preventing over-investigation and alleviating patient anxiety. The literature describes a multitude of causes for haemospermia, although many of these are not commonly found after investigation. However, men may be stratified into higher-risk groups according to several factors including: age > 40 years, recurrent or persistent haemospermia, the actual risk for PCa (e.g., positive family history), and concurrent haematuria. Based upon the literature, a management algorithm is proposed (Figure 9) [974,977,993,994].
Figure 9: Management algorithm for haemospermia 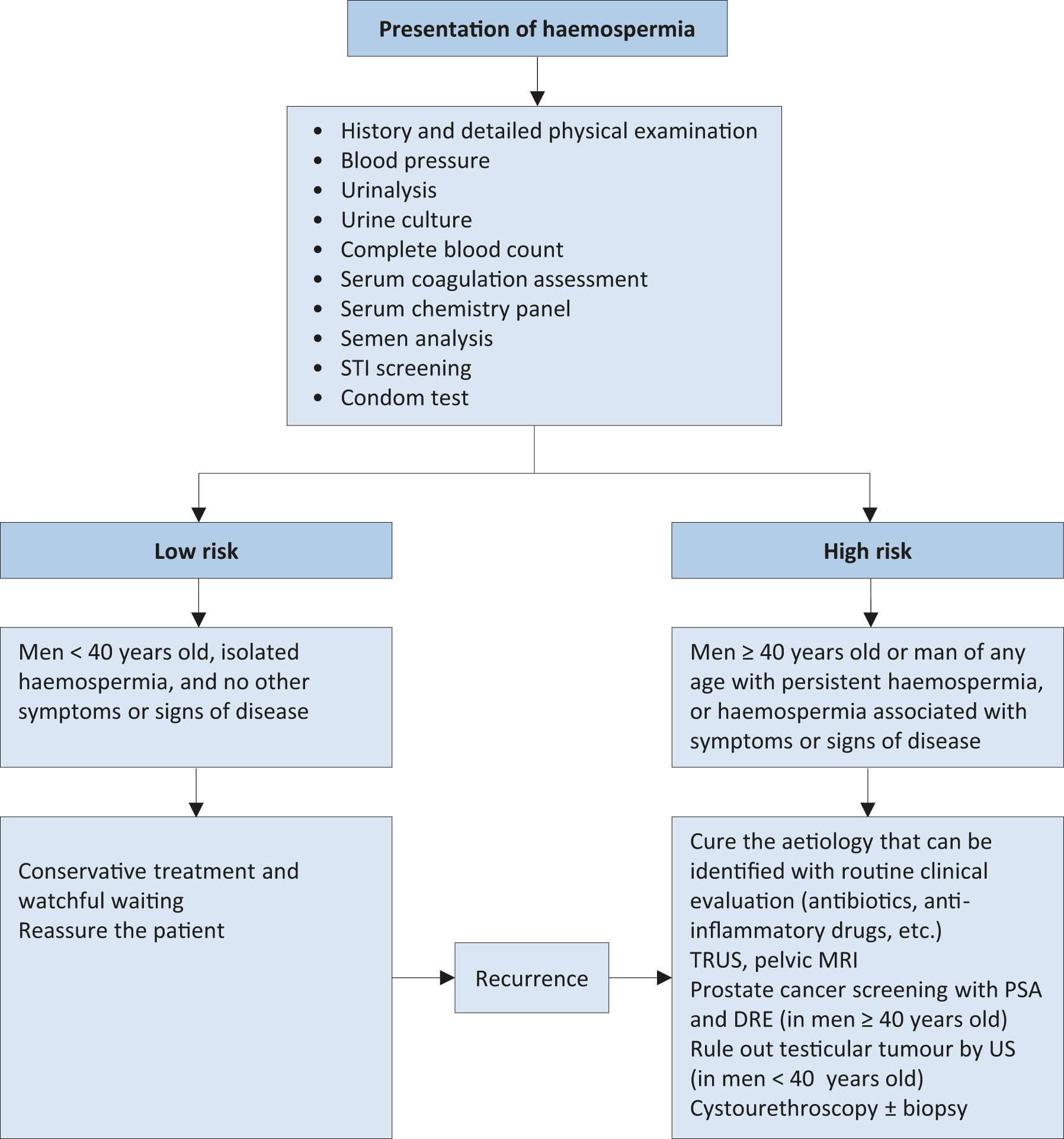 STI = Sexually transmitted infections; PSA = Prostate specific antigen; DRE = Digital rectal examination;US = Ultrasonography; TRUS = Transrectal ultrasonography; MRI = Magnetic resonance imaging.
STI = Sexually transmitted infections; PSA = Prostate specific antigen; DRE = Digital rectal examination;US = Ultrasonography; TRUS = Transrectal ultrasonography; MRI = Magnetic resonance imaging.
6.9. Recommendations for the management of recurrent haemospermia
Recommendations | Strength rating |
Perform a full medical and sexual history with detailed physical examination. | Strong |
Screen men aged > 40 years with persistent haemospermia for prostate cancer. | Weak |
Consider non-invasive imaging modalities (TRUS and MRI) in men aged > 40 years or men of any age with persistent or refractory haemospermia. | Weak |
Consider invasive methods such as cystoscopy and vesiculoscopy when the non-invasive methods are inconclusive or in patients with recurrent haemospermia. | Weak |