3. MALE HYPOGONADISM
3.1. Epidemiology and prevalence of male hypogonadism
Male hypogonadism is associated with decreased testicular function, with decreased production of androgens and/or impaired sperm production [5]. This is caused by impaired testicular function or as a result of inadequate stimulation of the testes by the hypothalamic-pituitary axis. Several congenital or acquired disorders causing impaired action of androgens are also described [5]. Hypogonadism may adversely affect multiple organ functions and quality of life (QoL) [5,6]. Late-onset hypogonadism (LOH) is a clinical condition in ageing men, which, by definition, must comprise both persistent specific symptoms and biochemical evidence of testosterone deficiency [5,7]. Late-onset hypogonadism is frequently diagnosed in the absence of an identifiable classical cause of hypogonadism, which becomes more prevalent with age, usually occurring, but not exclusively, in men aged > 40 years.
Male hypogonadism has also been called Testosterone Deficiency. The Panel has agreed to use the term Male Hypogonadism, which may better reflect and explain the underlying pathophysiology. Likewise, the Panel has further agreed to continue with the term testosterone therapy. The present Guidelines specifically address the management of adult male hypogonadism also called LOH. Some insights related to congenital or pre-pubertal hypogonadism are also provided and summarised.
The prevalence of hypogonadism increases with age and the major causes are central obesity, other co-morbidities (e.g., diabetes) and overall poor health [8]. In healthy ageing men, there is only a small gradual decline in testosterone; up to the age of 80 years, aging accounts for a low percentage of hypogonadism [8]. In men aged 40-79 years, the incidence of symptomatic hypogonadism varies between 2.1 and 5.7% [9-11]. The incidence of hypogonadism has been reported to be 12.3 and 11.7 cases per 1,000 people per year [9,12].
There is a high prevalence of hypogonadism within specific populations, including patients with type 2 diabetes (T2DM), metabolic syndrome (MetS), obesity, cardiovascular disease (CVD), chronic obstructive pulmonary disease (COPD), renal disease and cancer [11]. Low testosterone levels are common in men with T2DM [13] and a high prevalence of hypogonadism (42%) has been reported in T2DM patients [14].
Klinefelter syndrome, a trisomy associated with a 47,XXY karyotype, is the most prevalent genetic cause of primary hypogonadism (hypergonadotropic hypogonadism), with a global prevalence of 1/500-1,000 live male births [15-18]. However, < 50% of individuals with Klinefelter syndrome are diagnosed throughout their lifetime [19].
3.1.1. Body Composition and Metabolic Profile
Low testosterone levels are common in men with obesity. Male hypogonadism is associated with a greater percentage of fat mass and a lesser lean mass compared to men with adequate testosterone levels [20]. There is much evidence that a low testosterone level is strongly associated with increased visceral adiposity, but it also leads to lipid deposition in the liver and muscle and is associated with atherosclerosis [20]. In vitro studies have suggested that hypogonadism impairs glucose and triglyceride uptake into subcutaneous fat depots [20]. This enhances the uptake of glucose and triglycerides into ectopic fat depots.
Testosterone therapy has been associated with a reduced percentage of body fat and increase of lean body mass [21]. Data from a registry study have suggested that over a period of 11 years, testosterone therapy with long-acting intramuscular testosterone undecanoate was associated with a substantial but gradual loss of weight, along with a reduction in waist circumference [22]. Testosterone also reduces liver fat content and muscle fat storage [20].
3.1.2. Metabolic Syndrome/Type 2 Diabetes
Metabolic Syndrome is characterised by several specific components, including increased waist circumference, dyslipidaemia, hypertension, and impaired glucose tolerance. Hypogonadism is associated with central obesity, hyperglycaemia, insulin resistance and dyslipidaemia [low high-density lipoprotein (HDL)] cholesterol, raised total and low-density lipoprotein (LDL) cholesterol and triglycerides], hypertension and predisposition to T2DM, which are all components of MetS [23].
Several randomised controlled trials (RCTs) have shown that testosterone therapy might improve insulin resistance and hyperglycaemia and lower total and LDL-cholesterol [24-29]. Testosterone therapy in hypogonadal T2DM improved glycaemic control in some RCTs and registry trials; however, there is no conclusive evidence and the available data are still too limited to draw final conclusions [25,30,31]. A recent large placebo-controlled RCT, including 1,007 patients with impaired glucose tolerance or newly-diagnosed T2DM and total testosterone < 14 nmol/L, showed that testosterone therapy for 2 years reduced the proportion of patients with T2DM regardless of a lifestyle programme [29]. Similarly, a previous registry study reported that testosterone therapy was associated in time with remission of T2DM [30]. HDL-cholesterol may decrease, remain unchanged or increase with testosterone therapy. Testosterone therapy in men with MetS and low testosterone has been shown to reduce mortality compared to that in untreated men [32,33], although no conclusive evidence is available.
Erectile dysfunction (ED) is common in men with MetS and T2DM (up to 70% of patients). The causes of ED are multi-factorial and 30% of men with ED have co-existing testosterone-deficiency/hypogonadism. Some evidence has suggested that for patients with T2DM this is only found in men with clearly reduced testosterone levels (< 8 nmol/L or 2.31 ng/mL) [34]. From a pathophysiological point of view, it has been reported that this is because ED is predominantly caused by vascular and neuropathic disease, and therefore not likely in men who do not have established vascular disease. Therefore, men presenting with ED should be screened for MetS. Likewise, patients with ED and diabetes may be offered testosterone measurement.
Placebo-controlled RCTs of testosterone therapy in T2DM have demonstrated improved sexual desire and satisfaction, but not erectile function [25,34]. Similar results were recently derived from a meta-analysis of the available trials [35]. Accordingly, a large two-year RCT of testosterone undecanoate vs. placebo showed that testosterone therapy significantly improved sexual function and ED in men with impaired glucose tolerance or newly diagnosed T2DM low testosterone (< 14 nmol/L) [29].
3.2. Physiology of testosterone production
The pituitary gland regulates testicular activity through secretion of luteinising hormone (LH), which regulates testosterone production in Leydig cells and follicle-stimulating hormone (FSH), which mainly controls sperm production in seminiferous tubules [36,37]. The production and secretion of gonadotropins is stimulated by hypothalamic gonadotropin releasing hormone (GnRH) and inhibited by negative feedback mediated by the central action of sex steroids and inhibin B (Figure 1) [36,37]. Gonadotropin releasing hormone is secreted in a pulsatile manner and negatively controlled by the activity of hypothalamic neurons, including corticotrophin-releasing hormone (CRH) and β endorphin neurons [36,37]. Conversely, kisspeptin-1 (Kiss-1) neurons, neurokinin-B and tachykinin-3 are involved in GnRH stimulation. Leptin is involved in activation of Kiss-1 signalling [38]. About 25 mg of testosterone is present in the normal testes, and, on average, 5-10 mg of testosterone are secreted daily [36,37]. The testes also produce lesser amounts of other androgens, such as androstenedione and dihydrotestosterone (DHT). A small amount of extra-gonadal testosterone is derived from the circulating weak adrenal androgen precursor dehydroepiandrosterone (DHEA), although its specific contribution to daily testosterone production is limited in men [39,40]. In physiological terms, DHT formation accounts for 6-8% of testosterone metabolism, and the ratio of plasma testosterone/DHT is approximately 1:20 [36,37]. Finally, testosterone and its precursor, Δ4 androstenedione, can be aromatised through P450 aromatase to other bioactive metabolites, such as oestrone (E1) and 17-β-oestradiol (E2), with a daily production of ~45 μg [36,37]. Leydig cells, can also directly produce and release into the bloodstream small amounts of oestrogens, with a daily production rate of 5-10 μg (up to 20% of circulating oestrogens) [41].
3.2.1. Circulation and transport of testosterone
In healthy men, 60-70% of circulating testosterone is bound to the high-affinity sex-hormone-binding globulin (SHBG), a protein produced by the liver, which prevents its bound testosterone sub-fraction from biological action. The remaining circulating testosterone binds to lower affinity, high-capacity binding proteins, (albumin, α-1 acid glycoprotein and corticosteroid-binding protein), and only 1-2% of testosterone remains non-protein bound [42]. There is a general agreement that testosterone bound to lower-affinity proteins can easily dissociate in the capillary bed of many organs, accounting for so-called ‘bioavailable’ testosterone [42]. It is important to recognise that several clinical conditions and ageing itself can modify SHBG levels, thus altering circulating total testosterone levels (Table 1). Therefore, if not recognised, these factors could lead to an incorrect estimation of male androgen status. Therefore, when indicated (Table 1), SHBG should be tested and free testosterone calculated.
Figure 1: Physiology of testosterone production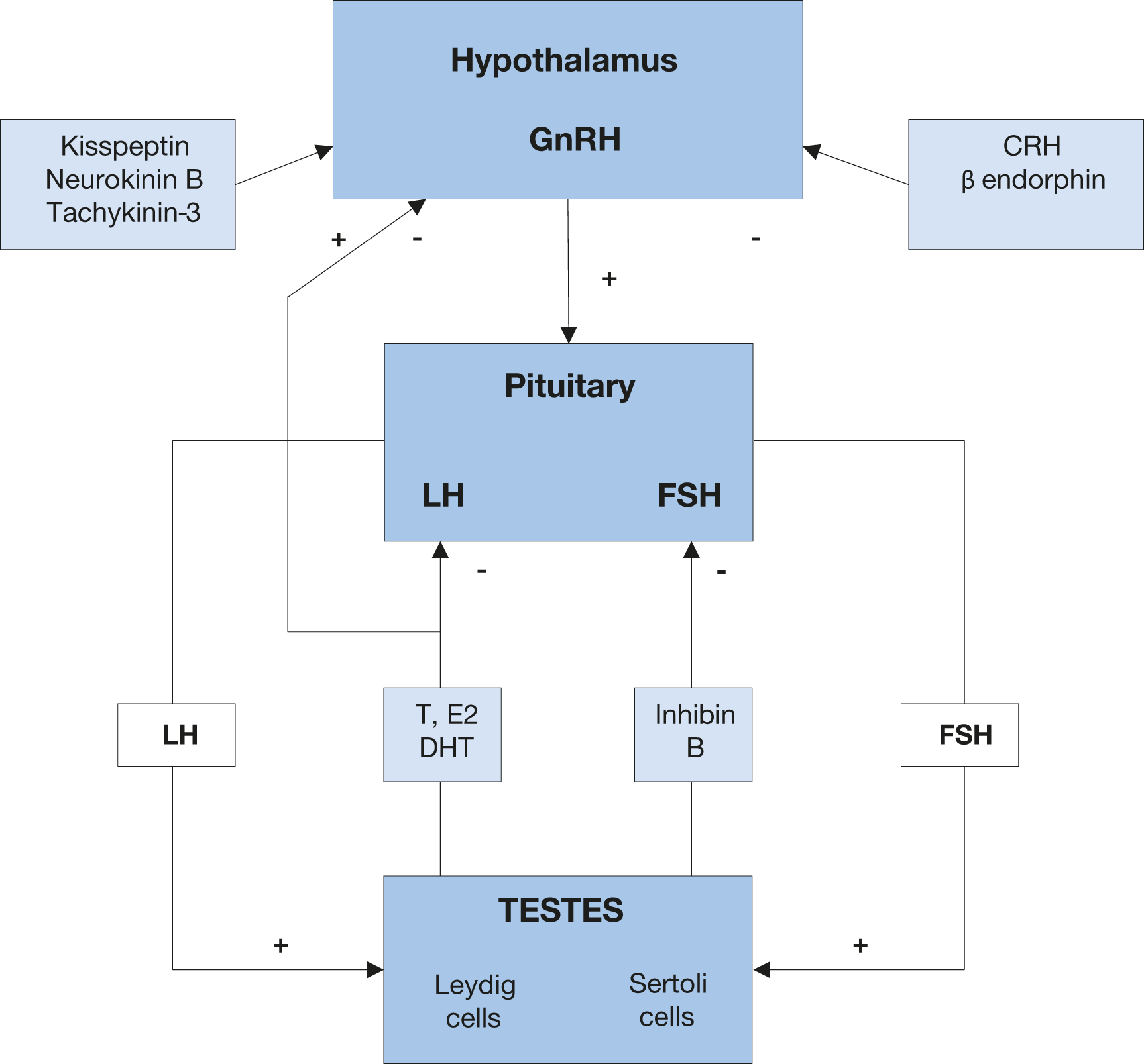 GnRH = gonadotropin releasing hormone; LH = luteinising hormone; FSH = follicle-stimulating hormone;T = testosterone; E2 = 17-β-estradiol; DHT = dehydroepiandrosterone; CRH = corticotrophin releasing hormone.
GnRH = gonadotropin releasing hormone; LH = luteinising hormone; FSH = follicle-stimulating hormone;T = testosterone; E2 = 17-β-estradiol; DHT = dehydroepiandrosterone; CRH = corticotrophin releasing hormone.
Table 1: Main factors associated with an increase or reduction of SHBG circulating levels
Main factors associated with an increase or reduction of SHBG circulating levels | |
SHBG increase | • Drugs: anticonvulsants, oestrogens, thyroid hormone • Hyperthyroidism • Hepatic disease • Ageing • Smoking • AIDS/HIV |
SHBG decrease | • Drugs: growth hormone (GH), glucocorticoids, testosterone, anabolic androgenic steroids • Hypothyroidism • Obesity • Acromegaly • Cushing’s disease • Insulin resistance (MetS/T2DM) • Non-alcoholic fatty liver disease (NAFLD), • Nephrotic syndrome |
3.2.2. Androgen receptor
Testosterone and DHT exert their biological action through activation of a specific nuclear receptor. The androgen receptor (AR) gene is localised on the X chromosome (Xq11–12), encoded in eight exons [43]. Exon 1 includes two polymorphic trinucleotide repeat segments encoding polyglutamine (CAG) and polyglycine (GGN) tracts in the N-terminal transactivation domain of its protein. Activity of the AR is inversely associated with the length of the CAG repeat chains [43]. However, the specific role of AR CAG repeat number in relation to hypogonadal symptoms or to clinical management of testosterone deficiency remains unclear [44,45]. A RCT has shown that a higher CAG repeat number is positively associated with a change in fasting insulin, triglycerides and diastolic blood pressure, demonstrating the more sensitive the receptor, the greater the benefit [46].
3.3. Role of testosterone in male sexual and reproductive health
3.3.1. Sexual development and maturation
Testosterone production in the foetal testis starts between the eighth and ninth week of gestation after the expression of the SRY gene, which regulates organisation of the undifferentiated gonadal ridge into the testis [47]. During the first trimester, the testes drive the virilisation of internal and external genitalia through placental human chorionic gonadotropin (hCG)-stimulated androgen secretion by Leydig cells. During foetal life, testosterone mainly controls the differentiation of internal genitalia and testicular descent (regression of gubernaculum testis), whereas DHT is mainly involved in the development of the external male genitalia [48]. During puberty, reactivation of the hypothalamus–pituitary-gonadal (HPG) axis allows the development of secondary sexual characteristics, spermatogenesis maturation and, along with the contribution of other hormonal axes, completion of the adolescent growth spurt [5,49]. Clinical models of aromatase deficiency and oestrogen receptor insensitivity have demonstrated that testosterone conversion to E2 is essential for epiphyseal closure and growth arrest [50].
3.3.2. Sexual function
Testosterone is involved in the regulation of all steps of the male sexual response. Sexual thoughts and motivations are universally accepted as the most testosterone-dependent aspects of male sexual behaviour [35,51,52]. The European Male Aging Study (EMAS), a population-based survey including 3,369 subjects aged 40-79 years from eight European countries, showed that sexual symptoms, particularly impairment of sexual desire, ED and decreased frequency of morning erections, were the most specific symptoms associated with age-depended decline of testosterone [10]. Similar findings were reported in patients consulting for sexual dysfunctions [53]. Accordingly, several brain areas, including the amygdala, medial preoptic area, paraventricular nucleus of the hypothalamus, and peri-aqueductal grey matter express androgen receptors [53,54]. Experimental and clinical studies have both documented that testosterone plays a crucial role in regulating penile function. In particular, testosterone controls the structural integrity necessary for penile erection, as well as several enzymatic activities within the corpus cavernosum, including a positive action on nitric oxide (NO) formation and a negative influence on the activity of the Ras homolog gene family member A/Rho-associated kinase (RhoA/ROCK) pathways [53,55]. Testosterone is also involved in the penile adrenergic response and cavernous smooth muscle cell turnover [53,55]. Although some authors have suggested a positive role for testosterone in regulating penile phosphodiesterase 5 (PDE5) expression and activity, others have shown an inhibitory role of oestrogens on this pathway [53,56].
More limited evidence has indicated a possible role of testosterone in regulating ejaculation, acting either at the central or peripheral level. Androgen receptors are expressed in several central spinal and super-spinal areas involved in the control of the ejaculatory reflex [57]. Additionally, the male genital tract expresses NO-PDE5 and RhoA/ROCK pathways, which are modulated by testosterone [57].
3.4. Classification and causes of male hypogonadism
Male hypogonadism can be classified according to the origin of the underlying problem into primary hypogonadism, if a consequence of testicular dysfunction, or secondary hypogonadism, if due to a pituitary or hypothalamic dysfunction (Table 2).
Primary hypogonadism is also called hypergonadotropic hypogonadism, since the pituitary tries to compensate for testicular dysfunction by increasing central stimulation. Conversely, in secondary hypogonadism the testes are inadequately stimulated by gonadotropins, usually with inappropriately normal or reduced gonadotropin levels [5,37]. A compensated or subclinical form of hypogonadism, characterised by normal testosterone serum levels and elevated LH production, has also been reported [58]; the clinical significance of the latter condition is unclear [58-61]. Finally, hypogonadism can also result from several conditions leading to reduced sensitivity/insensitivity to testosterone and its metabolites [5,37] (Table 2). This classification, based on the aetiology of hypogonadism, allows clinicians to adequately select appropriate treatment. In patients with secondary hypogonadism, both fertility and testosterone normalisation can be theoretically achieved with adequate treatment whereas in primary hypogonadism only testosterone therapy can be considered, which eventually impairs fertility due to suppression of the HGP axis [5,37] (Table 2). However, it should also be recognised that symptoms and signs of hypogonadism can be similarly independent of the site of origin of the disease. Conversely, the age of onset of hypogonadism can influence the clinical phenotype [38]. Accordingly, when the problem starts early, such as during foetal life, clinical phenotype can span from an almost complete female phenotype (e.g., complete androgen insensitivity or enzymatic defects blocking androgen synthesis) to various defects in virilisation. In the case of a pre- or peri-pubertal appearance of hypogonadism due to a milder central (isolated hypogonadotropic hypogonadism [HH]) or a peripheral defect (such as in Klinefelter’s syndrome), there might be delayed puberty with an overall eunuchoid phenotype. Finally, when hypogonadism develops after puberty and especially with ageing (i.e., LOH; see below), symptoms can be mild, and often confused the with ageing process per se [5,38].
In 2017, Grossmann and Matsumoto suggested a new classification of adult male hypogonadism, distinguishing functional versus organic hypogonadism [62]. Accordingly, organic hypogonadism is characterised by any proven pathology affecting the HPG axis and should be treated with conventional medication (i.e., gonadotropins or testosterone therapy). Conversely, functional hypogonadism is based on the absence of any recognised organic alterations in the HPG axis and should be treated first by resolving or improving the associated comorbidity. These Guidelines refer to the validated international classification of adult male hypogonadism.
Studies suggest that although men and women show similar prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induced disease (COVID-19), men usually present with worse outcomes when compared to females [63]. The possible mechanisms underlying this sex disparity are still not completely understood. The demonstration that testosterone can modulate the tissue expression of angiotensin-converting enzyme 2 (ACE2) and the transcription of a transmembrane protease, serine 2 (TMPRSS2), both involved in the process of virus-cellular internalisation, suggests the possibility that androgens play a role in explaining the aforementioned sex differences [64,65]. Overall, data seem to suggest that low rather than elevated circulating testosterone levels are more frequently associated with worse clinical outcomes in men with COVID-19
[66-73]. Accordingly, a meta-analysis suggested that reduced testosterone levels detected at hospital admission are associated with a four- or five-fold increased risk of being admitted to the Intensive Care Unit (ICU) or dying, after the adjustment for potential confounders [74]. In addition, Dhinsda et al., using two large academic health systems databases including 723 men with a history of COVID-19 reported that hypogonadal men had a higher risk of being hospitalised [75]. This is not surprising since ACE2 is expressed in several tissues including the testis and the ACE2 receptor has been demonstrated to be present on seminiferous duct cells, as well as on spermatogonia and on Leydig and Sertoli cells, with SARS-CoV-2 potentially contributing to impaired testosterone and sperm production [74,76,77]. Furthermore, it has also been suggested that the virus can result in a local intense inflammatory reaction in the testis thus supporting the development of a viral orchitis, eventually evolving into a vasculitis or to an autoimmune response, which can contribute to testis damage and impaired testosterone production [76,77].
Whether or not low testosterone can directly contribute to worse COVID-19 outcomes is still under investigation. The possibility that low testosterone in the acute phase of the virus infection can represent as an adaptive and resilient mechanism to mitigate an external insult by turning off testosterone-dependent functions, including reproduction and/or physical and sexual activity, which are not required when the physical condition is worsening, cannot be excluded [78,79]. Accordingly, a meta-analysis showed that secondary or mixed hypogonadism is more frequently observed in the acute phase of the infection [74]. Similar considerations have been proposed for LOH [80]. Studies evaluating subjects in the recovery phase of COVID-19 have documented either restored [81] or persistently low testosterone levels in the majority of cases [82]. Interestingly, in their longitudinal evaluation during the recovery phase, Salonia et al., showed that a further improvement of testosterone levels can be observed up to 12 months after COVID-19 [83]. The same authors suggested that associated morbidities can modulate testosterone recovery, supporting a secondary rather than a primary role of low testosterone in the COVID-19 negative outcomes [82]. However, although no information on the role of testosterone therapy in the acute phase of the disease is available yet, Dhinsda
et al., showed that the hypogonadal patients under testosterone therapy had a reduced risk to be hospitalised after SARS-CoV-2 infection [75]. Nevertheless, male subjects who have recovered from COVID-19 should be accurately followed-up to exclude any long-term andrological consequences including impairment in sperm and testosterone production [74].
Table 2: Classification of male hypogonadism
PRIMARY HYPOGONADISM (hypergonadotropic hypogonadism) | |
Congenital or developmental disorders | |
Common causes | Uncommon causes |
Klinefelter syndrome | - Rare chromosomal abnormalities - XX male syndrome - 47 XYY syndrome - 48 XXYY syndrome - 21 Trisomy (Down syndrome) - Noonan syndrome - Autosomal translocations1 - Defects of testosterone biosynthesis - CAH (testicular adrenal rest tumours) - Disorders of sex development (gonadal dysgenesis) - LHR gene mutations - Myotonic dystrophy (including type I and II) - Uncorrected cryptorchidism (including INSL3 and LGR8 mutations) - Bilateral congenital anorchia - Sickle cell disease - Adreno-leukodystrophy |
Acquired disorders | |
Drug-induced | Localised problems |
- Chemotherapy agents - Alkylating agents - Methotrexate - Testosterone synthesis inhibitors - Ketoconazole - Aminoglutethimide - Mitotane - Metyrapon | - Bilateral surgical castration or trauma - Testicular irradiation - Orchitis (including mumps orchitis) - Autoimmune testicular failure - Testicular Torsion - Alcohol/Cirrhosis - Environmental Toxins |
Systemic diseases/conditions with hypothalamus/pituitary impact | |
- Chronic systemic diseases* - Chronic organ failure* - Glucocorticoid excess (Cushing syndrome)* - Aging* - HIV | - Malignancies - Lymphoma - Testis cancer - Spinal cord injury - Vasculitis - Infiltrative diseases (amyloidosis; leukaemia) |
SECONDARY HYPOGONADISM (hypogonadotropic hypogonadism) | |
Congenital or developmental disorders | |
Common causes | Uncommon causes |
- Haemochromatosis* | - Combined hormone pituitary deficiency - Idiopathic hypogonadotropic hypogonadism - (IHH) with variants: - Normosmic IHH - Kallmann syndrome - Isolated LH β gene mutations - Prader-Willi Syndrome |
Acquired disorders | |
Drug-induced | Localised problems |
- Oestrogens - Testosterone or androgenic anabolic steroids - Progestogens (including cyproterone acetate) - Hyperprolactinaemia-induced drugs - Opiates - GnRH agonist or antagonist - Glucocorticoids | - Traumatic brain injury - Pituitary neoplasm (micro/macro-adenomas) - Hypothalamus tumours - Pituitary stalk diseases - Iatrogenic - Surgical hypophisectomy - Pituitary or cranial irradiation - Inflammatory and infectious diseases - Lymphocytic hypophysitis - Pituitary infections - Granulomatous lesions - Sarcoidosis - Wegener’s granulomatosis - Other granulomatosis - Encephalitis - Langerhans’ histiocytosis - Hyperprolactinaemia as a consequence of localised problems (hypothalamus-pituitary mass) |
Systemic diseases/conditions impacting the hypothalamus/pituitary | |
- Chronic systemic diseases* - Type 2 diabetes mellitus/Metabolic Syndrome/metabolic diseases - HIV infection - Chronic organ failure - Chronic Inflammatory Arthritis - Glucocorticoid excess (Cushing syndrome)* - Eating disorders* - Endurance exercise - Acute and critical illness - Ageing* | - Spinal cord injury - Transfusion-related iron overload (β-thalassemia) |
ANDROGEN RESISTANCE/DECREASED TESTOSTERONE BIOACTIVITY | |
Congenital or developmental disorders | |
- Aromatase deficiency - Kennedy diseases (spinal and bulbar muscular atrophy) and other extensions of CAG repeats - Partial or complete androgen insensitivity - 5α reductase type II (5αR) deficiency | |
Acquired disorders | |
Drug-induced | Localised problems |
- Drug-induced AR blockage - Steroidal antiandrogen - Cyproterone acetate - Spironolactone - Non-steroidal antiandrogen - Flutamide - Bicalutamide - Nilutamide - Drug-induced 5α reductase (5αR) activity blockade - Finasteride - Dutasteride - Drug-induced ER blockade - Clomiphene - Tamoxifen - Raloxifene - Drug-induced aromatase activity blockade - Letrozole - Anastrazole - Exemestane - Increased SHBG | - Coeliac disease |
* Conditions acting at central and peripheral levels resulting in either primary and secondary hypogonadism.
1 Different autosomal translocations can cause rare cases of hypogonadism and infertility.
3.5. Late-onset hypogonadism
Testosterone production declines with ageing. The EMAS study reported a 0.4% per annum (log hormone-age) decrease in total testosterone and a 1.3% per annum decline in free testosterone (fT) [8]. Late onset hypogonadism is the term frequently used to describe this phenomenon and the detection of hypogonadism in adulthood, in particular. Evidence has documented that several associated diseases and chronic co-morbidities can interfere with the HPG axis leading to development of primary hypogonadism or, more frequently, secondary hypogonadism in adulthood, thus significantly influencing the physiological age-dependent decline of testosterone. By combining the data from three different waves of the Massachusetts Male Aging Study (MMAS), a population-based, observational study including 1,709 men aged 40–70 years showed that associated comorbidity and obesity significantly decreased, whereas smoking tended to increase total, free and bio-available testosterone concentrations [84]. Similarly, data derived from the EMAS study confirmed these findings [8,59]. Based upon these data and other evidence, the concept of functional and organic hypogonadism has been more recently introduced [62]. The diagnosis of functional hypogonadism is based on the exclusion of a classical (organic) aetiology. The main causes of functional hypogonadism are obesity, co-morbidity and ageing with the first two accounting for most cases. Inflammatory cytokines released in chronic inflammation, and adipocytokines and oestradiol in obesity, can suppress the HPG axis. The role of ageing up to age 80 years seems relatively small [62]. Considering that suppression of HPG axis activity is functional, and potentially reversible by empiric measures, such as weight loss, the need for testosterone therapy has been questioned [62].
3.5.1. Diagnostic evaluation
The phenotype of the hypogonadal patient appears independent of the specific aetiology causing the problem, but it is more often affected by the age of onset of hypogonadism. When androgen deficiency is complete and develops during foetal life, symptoms can be dramatic, spanning from an almost complete female phenotype (complete androgen insensitivity or enzymatic defects blocking androgen synthesis) to various defects in virilisation and ambiguous genitalia (micropenis, hypospadias and cryptorchidism) [5,37]. Delay in puberty with an overall eunuchoid phenotype (scant body hair, high-pitched voice and small testes, penis and prostate) is typical of defects manifesting over the pre- or peri-pubertal period due to milder central (isolated HH) or peripheral defects (such as in Klinefelter syndrome) [5,37]. When hypogonadism occurs in adulthood, especially functional hypogonadism, symptoms can often be mild, difficult to recognise and frequently confused with the ageing process [5,37] or with chronic comorbidity. Several non-specific clinical features, such as fatigue, weakness, and decreased energy, as well as sexual impairment may be clinical manifestations. The EMAS study showed that a triad of sexual symptoms, including low libido, reduced spontaneous erections and ED, are typically associated with a decrease in serum testosterone levels [10]. Conversely, psychologic and physical symptoms were less informative [10].
The mainstay of LOH diagnosis includes signs and symptoms consistent with hypogonadism, coupled with biochemical evidence of low morning serum total testosterone levels on two or more occasions, measured with a reliable assay. Testosterone levels show a circadian variation, which persists in ageing men [85,86]. Likewise, testosterone levels are potentially influenced by food intake [87]; hence, serum total testosterone should be measured in fasting conditions and in the morning (between 07.00 and 11.00 hours). A confirmatory measurement should always be undertaken in the case of a primary pathological value, and certainly before starting any testosterone therapy.
Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) represents the gold standard and most accurate method for sex steroid evaluation; however, standardised automated platform immuno-assays for total testosterone assessment demonstrate a good correlation with LC-MS/MS [88]. Conversely, available immuno-assays are not able to provide an accurate estimation of fT; therefore, direct fT evaluation with these methods is not recommended and should be avoided [42]. Liquid chromatography-tandem mass spectrometry remains the standard method for fT determination. Alternatively, fT can be derived from specific mathematical calculations taking into account serum SHBG and albumin levels [89] (http://www.issam.ch/freetesto.htm).
Data from available meta-analyses have documented that testosterone therapy is ineffective when baseline levels are > 12 nmol/L (3.5 ng/mL). Positive outcomes are documented when testosterone levels are < 12 nmol/L, being higher in symptomatic patients with more severe forms of hypogonadism (< 8 nmol/L). Hence, 12 nmol/L should be considered as a possible threshold for starting testosterone therapy in the presence of hypogonadal symptoms [21,90]. As reported above, in the case of clinical conditions that may interfere with SHBG levels, evaluation of fT should be considered to better estimate actual androgen levels (Figure 2). Unfortunately, despite its potential clinical value [91], no validated thresholds for fT are available from clinical studies and this represents an area of uncertainty; however, some data indicate that fT levels < 225 pmol/L
(6.5 ng/dL) are associated with hypogonadal symptoms [10,53,92,93].
The determination of LH must be performed along with prolactin (PRL) when pathological total testosterone levels are detected, in order to correctly define the underlying conditions and exclude possible organic causes (Figure 2). Follicle-stimulating hormone determination can further support the diagnosis of primary or secondary hypogonadism [61,94]. Due to its negative influence on libido, PRL can also be considered as first-line screening in patients with reduced sexual desire. In addition, pituitary magnetic resonance imaging (MRI) scanning, as well as other pituitary hormone evaluations, is required in the presence of specific symptoms such as visual disturbances, headache [95,96] or when hyperprolactinemia is confirmed. Limited evidence suggests performing pituitary MRI also in the case of severe hypogonadism (< 6 nmol/L, 1.75 ng/mL) with inadequate gonadotropin levels (Figure 2) [95-97].
3.5.2. History taking
Specific symptoms associated with LOH are shown in Table 3. History of surgical intervention for cryptorchidism or hypospadias must be taken into account as possible signs of congenital defects. Likewise, chronic and systemic comorbidities must be comprehensively investigated in every patient. Use of drugs that potentially interfere with the HPG axis should be ruled out (Table 2). Acute diseases are associated with development of functional hypogonadism and determination of serum total testosterone levels should be avoided in these conditions. However, as detailed above, recent data derived from SARS-CoV-2 infected patients demonstrating worse outcomes in hypogonadal subjects suggests that the role of testosterone in the case of acute illness should be clarified [66,70,74,98]. Several self-reported questionnaires or structural interviews have been developed for screening of hypogonadism. Although these case-history tools have demonstrated clinical utility in supporting the biochemical diagnosis of hypogonadism, or in the assessment of testosterone therapy outcomes, their specificity remains poor and they should not be used for a systematic screening of hypogonadal men [99].
Figure 2: Diagnostic evaluation of Late-Onset Hypogonadism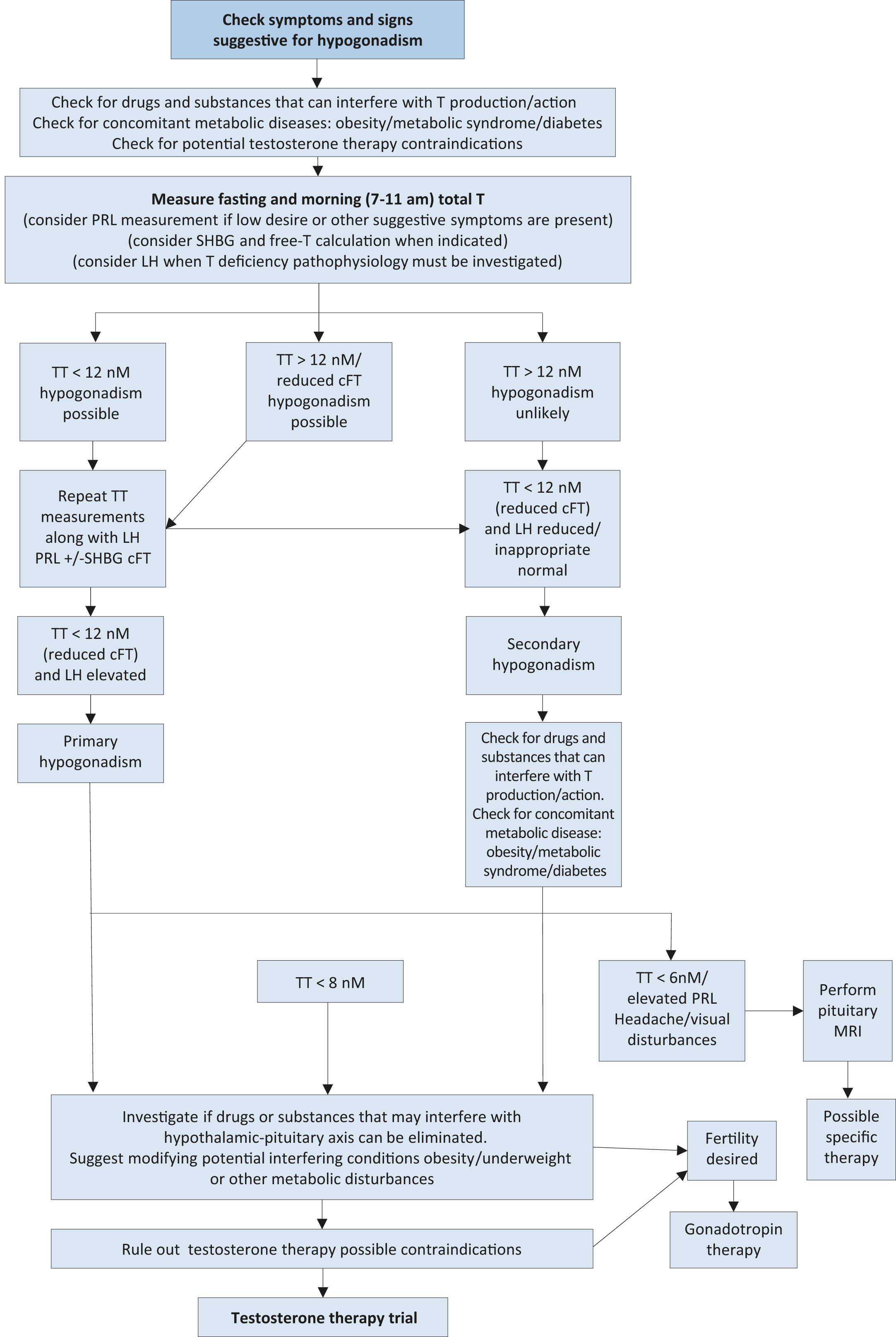 TT = total testosterone; cFT = calculated free testosterone; PRL = prolactin; SHBG = sex hormone-binding globulin; LH = luteinising hormone; MRI = Magnetic resonance imaging.
TT = total testosterone; cFT = calculated free testosterone; PRL = prolactin; SHBG = sex hormone-binding globulin; LH = luteinising hormone; MRI = Magnetic resonance imaging.
Table 3: Specific symptoms associated with LOH
Sexual symptoms | Physical symptoms | Psychological symptoms | |
More specific | - Reduced libido - Erectile dysfunction - Decreased spontaneous/morning erections | - Decreased vigorous activity - Difficulty walking > 1 km - Decreased bending | - Low mood/mood deflection - Decreased motivation - Fatigue |
Less specific | - Reduced frequency of sexual intercourse - Reduced frequency of masturbation - Delayed ejaculation | - Hot flushes - Decreased energy - Decreased physical strength/function/activity | - Concentration or mnemonic difficulties - Sleep disturbances |
3.5.3. Physical examination
Since obesity is frequently associated with hypogonadism (mostly functional), the determination of body mass index (BMI) and the measurement of waist circumference are strongly recommended in all individuals. Testicular and penile size, as well the presence of sexual secondary characteristics can provide useful information regarding overall androgen status. In addition, upper segment/lower segment ratio (n.v. > 0.92) and arm-span to height ratio (n.v. < 1.0) can be useful to identify a eunochoid body shape, especially in subjects with pre-pubertal hypogonadism or delayed puberty. Finally, digital rectal examination (DRE) should be performed in all subjects to exclude prostate abnormalities before testosterone therapy (any type) or to support suspicion of hypogonadism (in case of reduced volume) [100].
3.5.4. Summary of evidence and recommendations for the diagnostic evaluation of LOH
Summary of evidence | LE |
Sexual symptoms are the most specific symptoms associated with LOH. | 1a |
Diagnosis of LOH should be based on specific signs and symptoms of androgen deficiency, together with consistently low serum testosterone levels. | 1a |
12 nmol/L total testosterone (3.5 ng/mL) represents a reliable threshold to diagnose LOH. | 1a |
Functional hypogonadism is a consequence of comorbidity/concomitant drugs, which can impair testosterone production in adulthood. The diagnosis of functional hypogonadism is a diagnosis of exclusion, after ruling out organic causes of hypogonadism. | 4 |
Recommendations | Strength rating |
Check for concomitant diseases, drugs and substances that can interfere with testosterone production/action. | Strong |
Measure total testosterone in the morning (07.00 and 11.00 hours) and in the fasting state, with a reliable laboratory assay. | Strong |
Repeat total testosterone on at least two separate occasions when < 12 nmol/L and before starting testosterone therapy. | Strong |
Use 12 nmol/L total testosterone (3.5 ng/mL) as a reliable threshold to diagnose late onset hypogonadism (LOH). | Strong |
Consider sex hormone-binding globulin and free-testosterone calculation when indicated. | Strong |
Calculated free-testosterone of < 225 pmol/L has been suggested as a possible cut-off to diagnose LOH. | Weak |
Analyse luteinising hormone and follicle-stimulating hormone serum levels to differentiate between primary and secondary hypogonadism. | Strong |
Consider prolactin (PRL) measurement if low sexual desire (or other suggestive signs/symptoms) and low or low-normal testosterone is present. | Strong |
Perform pituitary magnetic resonance imaging (MRI) in secondary hypogonadism, with elevated PRL or symptoms specific of a pituitary mass and/or presence of other anterior pituitary hormone deficiency. | Strong |
Perform pituitary MRI in secondary severe hypogonadism (total testosterone < 6 nmol/L). | Weak |
3.5.5. Recommendations for screening men with LOH
Recommendations | Strength rating |
Screen for late onset hypogonadism (LOH) (including in T2DM) only in symptomatic men. | Strong |
Do not use structured interviews and self-reported questionnaires for systematic screening for LOH as they have low specificity. | Strong |
3.6. Treatment of LOH
3.6.1. Indications and contraindications for treatment of LOH
Patients with symptomatic hypogonadism (total testosterone < 12 nmol/L) without specific contraindications are suitable candidates to receive testosterone therapy (Table 4).
Absolute contraindications are untreated breast and prostate cancer (PCa). Acute cardiovascular events as well as uncontrolled or poorly controlled congestive heart failure and severe lower urinary tract symptoms (LUTS) International Prostate Symptom Score (IPSS) score > 19 represent other contraindications, as there is insufficient information on the long-term effects of testosterone therapy in these patients [66]. A positive family history for venous thromboembolism requires further analysis to exclude a condition of undiagnosed thrombophilia-hypofibrinolysis [101]. These patients need to be carefully counselled prior to testosterone therapy initiation. A haematocrit (HCT) > 54% should require testosterone therapy withdrawal, reduction in dose, change of formulation and venesection depending on the clinical situation to avoid any potential cardio-vascular complications. Lower baseline HTC (48-50%) should be carefully evaluated before testosterone therapy initiation, to avoid pathological increases during treatment, especially in high-risk men such as those with COPD or Obstructive Sleep Apnoea Syndrome (OSAS). Accordingly, the Framingham Heart Study showed that HCT > 48% represented a condition associated with increased risk of coronary artery disease (CAD) and mortality and was associated with cardiovascular disorders [102]. Finally, testosterone therapy suppresses gonadotropin and endogenous testosterone secretion as well as spermatogenesis [103]. Hence, testosterone therapy is contraindicated in individuals who desire fertility [104]. Secondary hypogonadism is characterised by low or inappropriately normal gonadotropin levels; therefore, the rationale is to substitute the gonadotropin deficiency with FSH and LH analogues, if fertility is desired [105].
Table 4: Main contraindications of testosterone therapy
Main contraindications of testosterone therapy | |
Absolute contraindications | Locally advanced or metastatic prostate cancer (PCa) Male breast cancer Men with an active desire to have children Haematocrit > 54% Uncontrolled or poorly controlled congestive heart failure |
Relative contraindication | IPSS score > 19 Baseline haematocrit 48-50% Familial history of venous thromboembolism |
3.6.2. Testosterone therapy outcomes
3.6.2.1. Sexual dysfunction
Sexual concerns are the main symptoms of the hypogonadal patient [5,10,106,107]. A consistent body of evidence shows that testosterone therapy in hypogonadal men (total testosterone < 12 nmol/L) may have a beneficial effect on several aspects of sexual life; in contrast, there is no evidence of benefits in using testosterone therapy for treating sexual dysfunction in eugonadal men [55,90,108,109]. The beneficial effect on sexual function seems to be more related to testosterone level normalisation than the specific testosterone formulations used [109,110].
A meta-analysis of placebo-controlled RCTs using the International Index of Erectile Function (IIEF) [111] as a possible tool for outcome evaluation, showed that testosterone therapy significantly improves erectile function (as measured by IIEF-Erectile Function domain score) and that patients with more severe hypogonadism (i.e., total testosterone < 8 nmol/L) are more likely to achieve better improvement than patients with milder hypogonadism (i.e., total testosterone < 12 nmol/L). Similar results were observed for sexual desire; however, the presence of metabolic comorbidity (such as diabetes and obesity) decreased the magnitude of these improvements. In particular, testosterone therapy alone resulted in a clinically effective outcome only in patients with milder ED [90]. Similar results have also been confirmed in an update analysis [112]. Other sexual function parameters, such as intercourse, orgasm and overall satisfaction, were all improved compared with placebo [90,112]. Men with comorbidity such as diabetes usually show modest improvements in terms of sexual function after testosterone therapy and may potentially require concomitant phosphodiesterase type 5 inhibitors (PDE5Is) to improve effectiveness [5,109,113]. A meta-analysis including 913 patients derived from eight RCTs suggested that combination therapy (testosterone and PDE5I) was superior when compared to PDE5I alone in improving erectile function [114]. However, due to methodological limitations of the included RCTs further studies on combination treatment with testosterone therapy and PDE5Is are required. Currently, the specific beneficial effect derived from the combined use of testosterone therapy and PDE5Is is not completely clear [55]. Similarly, information related to the combined use of testosterone therapy with other ED drug therapies is lacking [5,109].
The Sexual Function Trial of the Testosterone Trials (TTrials) (one of the largest placebo-controlled trials on testosterone therapy) documented consistent improvements in 10 of 12 measures of sexual activities in older
(> 65 years) hypogonadal men, particularly in frequency of intercourse, masturbation and nocturnal erections (as measured by PDQ-Q4) [115]. The magnitude in improvement was shown to be proportional to the increase in serum total testosterone, fT and E2 levels, it was not possible to demonstrate a threshold level [116]. A study of 220 men with MetS with or without T2DM also found that sexual function improved in men who reported sexual problems with improvement in IIEF scores with specific increases in libido and sexual satisfaction [25].
3.6.2.2. Body composition and metabolic profile
Late onset hypogonadism is associated with a greater percentage of fat mass and a lesser lean mass compared to testosterone-replete men [93]. The major effect of low testosterone is to increase visceral adiposity but it also leads to deposition of lipids in the liver and muscle and is associated with atherosclerosis [20]. Some published data have suggested that testosterone therapy reduces percentage body fat and increases lean mass [117]. Testosterone therapy has also been found to decrease waist circumference, body weight and BMI, with these effects more predominant after 12 months of treatment [117-119]. Over two years, the T4DM RCT reported that men on testosterone therapy and a lifestyle programme had a greater reduction in waist circumference, total and abdominal fat mass and an increase in total and arm muscle mass and an increased strength in the non-dominant hand compared to a lifestyle programme alone [29]. There was a trend toward reduction in body weight although this approached significance but did not reach significance. The latter result is probably compounded by the increase in muscle mass as well as the decrease in fat mass. However, it should be recognised that the results of previous studies are mainly derived from registry and observational trials, which have important limitations due to the risk of selection bias for the non-random assignment of testosterone exposure. Accordingly, data derived from RCTs showed only an improvement of fat mass and lean mass of the same amount without any modifications in body weight [21]. A meta-analysis including seventeen RCTs specifically investigated the role of testosterone therapy on several metabolic parameters in patients with T2DM and/or MetS [35]. In line with what was reported in the general population, testosterone therapy was associated with an improvement in body composition either in T2DM or MetS without any effects on body weight. Similarly positive effects were also observed on fasting glycemia and insulin resistance (HOMA index) whilst more conflicting data were obtained for HbA1c and lipid profile [35].
3.6.2.3. Mood and cognition
Several observational studies have documented a relationship between depressive symptoms, reduced QoL and hypogonadism [120,121]. However, the specific relationship between hypogonadism and the incidence of depression is still unclear [121]. Only a few placebo-controlled RCTs have investigated the role of testosterone therapy in improving depressive symptoms. Data derived from TTrials showed that testosterone therapy improved mood, and depressive symptoms as continuous measures using several instruments [115]. However, the final effect was small in magnitude. In line with these data, the largest meta-analysis of available studies, including 1,890 hypogonadal men (baseline total testosterone < 12 nmol/L or fT < 225 pmol/L) men from 27 RCTs, documented that the positive effect of testosterone therapy was particularly evident in patients with milder symptoms [122]. The BLAST study of testosterone therapy in T2DM reported that those men with depression were less likely to respond with regards to symptoms of sexual dysfunction compared to men without depression [31].
Robust data on the effect of testosterone therapy on QoL are limited. Although recent meta-analyses suggest a significant effect of testosterone therapy over placebo, the magnitude is low and the heterogeneity high, therefore reducing the scientific value of the effect [110,123].
The role of testosterone therapy in patients with cognitive impairment is even more uncertain. The TTrials evaluated the effect of testosterone therapy in 493 individuals with age-associated memory impairment in order to assess possible improvement of several aspects of cognitive function. However, the final results failed to demonstrate any beneficial effect of testosterone therapy in improving cognitive function [115].
3.6.2.4. Bone
Evidence suggests that bone mineralisation requires circulating sex steroids within the normal range [124]. The possible association between mild hypogonadism and osteopenia/osteoporosis is weak, whereas severe hypogonadism (total testosterone < 3.5 nM) is frequently associated with bone loss and osteoporosis, independent of patient age [124]. Three independent meta-analyses showed a positive effect of testosterone therapy on bone mineral density (BMD), with the highest effect at the lumber level [125-127]. Interestingly, the this latter meta-analysis has provided novel evidence that the role of testosterone on BMD was even higher in patients with diabetes [127], who are themselves at a higher risk of hypogonadism and bone fracture [35,128,129]. Similarly, data derived from TTrials and the T4DM studies confirmed that testosterone therapy increased BMD in hypogonadal ageing men. The TTrial found increased BMD in trabecular bone at the lumbar level [115], whereas the T4DM study reported greater increases in cortical bone [130]. Changes in hip and spine BMD were similar in both studies. However, available data are insufficient to determine the effect of testosterone therapy alone on the risk of fractures [124]. The use of testosterone therapy as an adjunct to anti-resorptive treatment in hypogonadal patients at high risk of fractures has not been established. Therefore, anti-resorptive therapy must be the first-choice treatment in hypogonadal men at high risk for bone fractures. The combination of anti-resorptive treatment and testosterone therapy should be offered only in conjunction with hypogonadism-related symptoms.
3.6.2.5. Vitality and physical strength
The role of testosterone in stimulating muscle growth and strength is well established. Accordingly, androgenic-anabolic steroids (AAS) have been used as performance-enhancing agents to increase physical performance in competitive sport [131]. In this regard, testosterone therapy in hypogonadal men has been shown to increase muscle mass and reduce fat mass, with limited effects on final weight [21]. Despite this evidence, the role of testosterone therapy in older men with mobility limitations remains unclear. The National Health and Nutrition Examination Survey 1999-2004 [132] was unable to detect any association between overall circulating testosterone levels and the amount of physical activity. However, among non-obese men, those in the highest physical activity tertile were significantly less likely to have low or low-normal testosterone than those in the lowest tertile. Data from TTrials indicated that testosterone therapy did not substantially increase the fraction of men whose 6-minute walking distance increased > 50 m or the absolute increase in the distance walked by those enrolled in the physical function trial [115]. However, when the whole population of the TTrials was considered, a significant, although modest, positive effect on these two parameters was reported [115]. Similar data were derived from the Vitality Trial [115].
3.6.2.6. Summary of evidence and recommendations for testosterone therapy outcome
Summary of evidence | LE |
Testosterone therapy can improve milder forms of ED and libido in hypogonadal men. | 1a |
Testosterone therapy can improve other sexual symptoms, including intercourse frequency, orgasm and overall satisfaction. | 1b |
Testosterone therapy can similarly increase lean mass, reduce fat mass, and improves insulin resistance. | 1a |
Testosterone therapy can improve weight, waist circumference and lipid profile, but findings are not unique. | 3 |
Testosterone therapy can improve milder depressive symptoms in hypogonadal men. | 1a |
Testosterone therapy can improve bone mineral density, but information related to fracture risk is lacking. | 1a |
Recommendations | Strength rating |
Do not use testosterone therapy in eugonadal men. | Strong |
Use testosterone therapy as first-line treatment in patients with symptomatic hypogonadism and mild erectile dysfunction (ED). | Strong |
Use a combination of phosphodiesterase type 5 inhibitors and testosterone therapy in more severe forms of ED as it may result in better outcomes. | Weak |
Use conventional medical therapies for severe depressive symptoms and osteoporosis. | Strong |
Do not use testosterone therapy to reduce weight and enhance cardio-metabolic status. | Weak |
Do not use testosterone therapy to improve cognition vitality and physical strength in ageing men. | Strong |
3.6.3. Choice of treatment
3.6.3.1. Lifestyle factors
As reported above, functional hypogonadism is frequently associated with obesity and metabolic disorders [133]. Therefore, weight loss and lifestyle changes should be the first approach for all overweight and obese men with hypogonadism. A previous meta-analysis documented that a low-calorie diet is able to revert obesity-associated secondary hypogonadism by increasing total testosterone and fT, reducing oestrogens and restoring normal gonadotropin circulating levels [134]. This was confirmed in an updated meta-analysis showing that the increase in testosterone is significantly associated with weight reduction [135]. Similar results can be obtained through physical activity, which is associated with the duration of scheduled exercise and weight loss obtained [135]. However, it should be recognised that the increase in testosterone levels observed after a low-calorie diet and physical activity is small (1-2 nmol) [134,135]. It should also be recognised that 60-86% of weight lost is regained after 3 years and 75-121% after 5 years [136]. A greater testosterone increase can be achieved through bariatric surgery, which results in an average increase of about 10 nmol/L depending on the degree of weight loss [135]. Lifestyle changes represent an essential part of the management of obesity; however, some evidence suggests that when compared to lifestyle modifications alone, testosterone therapy-treated obese men benefit most from relief of their symptoms associated with testosterone deficiency, whereas those not treated did not benefit [105]. There is limited evidence to suggest that combination of life-style interventions and testosterone therapy in symptomatic hypogonadal men might result in better outcomes [93]. As described above, the T4DM study has demonstrated that over 2-years testosterone therapy with lifestyle intervention was superior to lifestyle intervention alone in reducing waist circumference and total and abdominal fat content. There was no significant reduction in body weight when compared to lifestyle intervention alone [29].
3.6.3.2. Medical preparations
Several testosterone formulations are available (Table 5). Direct comparisons among different testosterone products are still lacking. Candidates for testosterone therapy should be adequately informed about the possible risks and benefits of all available testosterone preparations. The final choice should be based on the clinical situation, testosterone formulation availability, and patient needs and expectations [137].
3.6.3.2.1. Oral formulations
The esterification of testosterone with a long-chain fatty acid (testosterone undecanoate; TU) enables testosterone to be absorbed by the intestine through the lymphatic system, by-passing liver metabolism. This formulation has been available in oleic acid since the 1970s, and it has been recently reformulated in a mixture of castor oil and propylene glycol laureate (TU caps), to allow the drug to be maintained at room temperature without degradation [137]. The main limitation is related to the poor bioavailability, which is strongly dependent on dietary fat content [137]. Recently, the US Food and Drug Administration (FDA) approved a new formulation of oral TU incorporating a liquid-filled soft gel capsule that comes in multiple capsule strengths (158 mg, 198 mg and 237 mg) and multiple dose strengths (158 mg, 198 mg, 237 mg, 316 mg and 396 mg), which improves oral availability [138]. An open label study of approximately 24 months’ duration (NCT02722278), including 161 hypogonadal men, showed that TU caps formulation resulted in a significant improvement of all sexual function domains at all time points when compared to baseline along with an excellent safety profile [139].
Mesterolone is a 5α-DHT derivate available for oral administration. Along with DHT, mesterolone cannot be converted to oestrogens and can only be used for a limited period and specific indications, such as the presence of painful gynaecomastia. However, the lack of a full spectrum of testosterone bioactivity strongly limits its long-term use [137].
3.6.3.2.2. Parenteral formulations
Injectable testosterone preparations can be classified according to their half-lives (Table 5). Testosterone propionate is a short-term ester formulation requiring multiple fractionated doses (usually 50 mg, every 2-3 days), thus representing a major limitation for its use [137]. Cypionate and enanthate-T esters are short-term formulations, requiring administration every 2-4 weeks. A formulation containing mixed testosterone esters (TU, isocaproate, phenyl propionate, propionate - Sustanon®) which allows some benefit of a smoother release of testosterone into the circulation is available in some countries. The use of these older formulations is associated with wide fluctuations in plasma testosterone concentrations and is often reported as unpleasant by patients and potentially resulting in adverse effects, such as polycythaemia [137,140]. A longer-lasting TU injectable formulation is widely available [137], with a good safety/benefit profile allowing the maintenance of normal stable testosterone levels at a dose of 1,000 mg initially every 12 weeks, following a 6-week loading dose, but can be adjusted to a frequency of 10-14 weeks dependent on the trough (pre-injection level) after 3-5 injections to maintain levels in the therapeutic range (usually > 12 and < 18 nmol/L) [137,141]
3.6.3.2.3. Transdermal testosterone preparations
Among the available transdermal formulations, testosterone gels represent the most frequently used preparations. The gel is quickly absorbed by the stratum corneum, creating a reservoir within the subcutaneous tissues from where testosterone is continuously delivered for 24 hours, after a single daily application. These formulations have been shown to normalise serum testosterone levels with an excellent safety profile [137]. The introduction of specific devices and skin enhancers has resulted in better skin penetration of the drugs, thus reducing potential adverse effects. Local skin adverse effects are limited when compared to those with traditional testosterone patches, but they potentially allow transference of testosterone during close contact with the skin surface. The risk can be reduced by wearing clothing or by applying the gel on skin surfaces not usually touched (e.g., the inner thigh surface) [137]. To reduce the total amount of gel applied and residual quantities remaining on the skin, new formulations of testosterone gel have been introduced with a testosterone concentration of 1.62-2% [137]. Another transdermal testosterone formulation includes a topical, alcohol-based testosterone (2%) solution, which must be applied to the underarm once daily, using a metered dose applicator [137]. This testosterone formulation is not available in Europe. Testosterone levels should be monitored to optimise the testosterone dose. Blood collection is best taken at 2-4 hours after gel application to use the peak level of testosterone absorbed as a reference for adequate therapeutic levels. Levels of testosterone after application can vary and a repeat measurement may be indicated especially as sometimes, inadvertently, the skin over the vene-puncture site can be contaminated by the gel, leading to falsely elevated results.
In some European countries, DHT is available as a hydroalcoholic 2.5% gel. It is rapidly absorbed, reaching a steady state in 2-3 days [137]. Similar to that reported for mesterolone, DHT is not aromatised but can be useful for treating particular conditions, such as gynaecomastia and microphallus [137].
3.6.3.2.4. Transmucosal formulations
3.6.3.2.4.1. Transbuccal testosterone preparations
A testosterone buccal system is still available in several countries. It consists of a sustained-release muco-adhesive buccal-testosterone-tablet requiring twice-daily application to the upper gums. The tablet does not dissolve completely in the mouth and must be removed after 12 hours. This formulation has been proven to restore testosterone levels within the physiological range with minimal or transient local problems, including gum oedema, blistering and gingivitis [137].
3.6.3.2.4.2. Transnasal testosterone preparations
A gel for intranasal administration is available in some countries, including the USA and Canada. It requires administration two or three times daily using a specific metered-dose pump. The application is rapid, non-invasive, convenient, and avoids secondary transference observed with other topical products [137].
3.6.3.2.5. Subdermal depots
The implantation of testosterone pellets, available in the USA, UK and Australia, represents the longest available testosterone formulation lasting from 4-7 months. However, the procedure is invasive and may be unattractive to patients [137].
3.6.3.2.6. Anti-oestrogens
Anti-oestrogens, including selective oestrogen receptor (ER) modulators (SERMs) and aromatase inhibitors (AI) have been suggested as off-label treatments to restore testosterone levels and fertility in men with functional secondary hypogonadism or idiopathic infertility. They work by preventing down-regulation of the HPG axis by oestrogens and, for this reason are particularly useful in men with obesity and metabolic disorders [135]. In the latter case, the hypothesis is that the excess of adipose tissue leads to increased aromatase activity and oestrogens levels resulting in impairment of the HPG [133]. Due to their putative mechanism of action, they require an intact HPG axis and cannot work in primary hypogonadism or secondary hypogonadism due to organic damage of the HPG axis. Both types of SERMs, which bind ERs with an agonist or antagonist effect depending upon the target tissue, and AIs, which prevent androgens from being converted into oestrogens by aromatase, have been used in clinical practice [137]. The evidence published so far is poor; all these products are off-label treatments and SERMs, due to their agonistic effect on venous vessels, could predispose men to the development of venous thromboembolism [137]. In this context patients should be warned of the potential increased risk of venous thromboembolism, although data are lacking. Long-term use of these agents can lead to reduced bone density and development of osteoporosis, potentially increasing fracture risk.
3.6.3.2.7. Gonadotropins
Considering the aforementioned limitations regarding the use of anti-oestrogens, gonadotropin therapy should be considered the standard in men with secondary hypogonadism who desire paternity (Table 5) [137]. The treatment is based on the use of human chorionic gonadotropin (hCG), purified from the urine of pregnant women. The most expensive recombinant hCG (rhCG) and LH (rLH) formulations do not offer clinical advantages [137]. According to a meta-analysis of the available evidence, hCG should be administered with FSH since combined therapy results in better outcomes. Similar to recombinant hCG, recombinant FSH (rFSH) does not seem to offer any advantages compared to urinary-derived preparations [141]. More details on the use of gonadotropins are provided in Section 10.
Table 5: Available preparations for hypogonadism treatment
Formulation | Chemical structure | t 1/2 | Standard dosage | Advantages | Disadvantages |
GONADOTROPINS | |||||
Human chorionic gonadotrophin (HCG) | |||||
Extractive | HCG purified from the urine of pregnant women | NA | 1,000-2,000 IU | Low cost | Multiple weekly administration |
Recombinant | Human recombinant HCG | NA | No data in men | NA | |
Luteotropic hormone (LH) | |||||
Recombinant | Human recombinant LH | NA | No data in men | NA | |
Follicle-stimulating hormone (FSH) | |||||
Extractive | FSH purified from urine of pregnant women | NA | 75-150 IU | Low cost | Multiple weekly administration |
Recombinant | Human recombinant FSH | NA | 75-150 IU | Multiple weekly administration | |
TESTOSTERONE PREPARATIONS | |||||
Oral | |||||
Testosterone undecanoate | 17-α-hydroxylester | 4 hours | 120-240 mg 2-3 times daily | - Reduction of liver involvement - Oral convenience - Modifiable dosage | - Unpredictable absorption depending on dietary fat content - Must be taken with meals |
Mesterolone | 1α-methyl-4, 5α-dihydro-testosterone | 12 hours | 50-100 mg 2-3 times daily | - Oral convenience - Modifiable dosage - Useful in gynaecomastia | - Not aromatisable |
Parental | |||||
Testosterone enanthate | 17-α-hydroxylester | 4-5 days | 250 mg every 2-3 weeks | - Low cost - Short-acting preparation allowing drug withdrawal in case of adverse effects | - Fluctuations in circulating testosterone levels - Multiple injections - Relative risk of polycythemia |
Testosterone cypionate | 17-α-hydroxylester | 8 days | 200 mg every 2-3 weeks | - Low cost - Short-acting preparation allowing drug withdrawal in case of adverse effects | - Fluctuations in circulating testosterone levels - Multiple injections - Relative risk of polycythemia |
Testosterone propionate | 17-α-hydroxylester | 20 hours | 100 mg every 2 days | - Low cost - Very short-acting preparation allowing drug withdrawal in case of adverse effects | - Fluctuations in circulating testosterone levels - Multiple injections - Relative risk of polycythemia |
Testosterone ester mixture Propionate (30mg) Phenylpropionate (60 mg) Isocaproate (60 mg) Decanoate (100 mg) | 4-androsten-3-one- 17 beta-hydroxy-androst-4-en-3-one | 4-5 days | 250 mg every 3 weeks | - Low cost - Short-acting preparation allowing drug withdrawal in case of adverse effects | - Fluctuations in circulating testosterone levels - Multiple injections - Relative risk of polycythemia |
Testosterone undecanoate in castor oil | 17-α-hydroxylester | 34 days | 1,000 mg every 10-14 weeks *750 mg every 10 weeks | - Steady-state testosterone level without fluctuation - Long-lasting - Less frequent administration | - Pain at injection site - Long-acting preparation not allowing rapid drug withdrawal in case of adverse effects |
Surgical implants | Native testosterone | -- | 4-6 200 mg implants lasting up to 6 months | - Long duration and constant serum testosterone level | - Placement is invasive - Risk of extrusion and site infections |
TRANSDERMAL | |||||
Testosterone patches | Native testosterone | 10 hours | 50-100 mg/day | Steady-state testosterone level without fluctuation | - Skin irritation - Daily administration |
Testosterone gel 1-2% | Native testosterone | 6 hours | 50-100 mg/day | Steady-state testosterone level without fluctuation | - Possible transfer during intimate contact - Daily administration |
Underarm testosterone (testosterone solution 2%) | Native testosterone | NA | 60-120 mg/day | Steady-state testosterone level without fluctuation | - Possible transfer during intimate contact - Daily administration |
Dihydro-testosterone gel 2.5% | Native dihydro-testosterone | NA | 34-70 mg/day | - Steady-state testosterone level without fluctuation - Useful in gynaecomastia | - Possible transfer during intimate contact - Daily administration - Not aromatisable |
TRANSMUCOSAL | |||||
Testosterone buccal system | Native testosterone | 12 hours | 60 mg | Steady-state testosterone level without fluctuation | - Possible oral irritation - Twice-daily dosing - Unpleasant taste |
Testosterone nasal | Native testosterone | 6 hours | 33 mg 3 times daily | Steady-state testosterone level without fluctuation | - Nasal irritation - Multiple daily administration |
NA = not applicable.
3.6.3.3. Summary of evidence and recommendations for choice of treatment for LOH
Summary of evidence | LE |
Weight loss obtained through a low-calorie diet and regular physical activity result in a small improvement in testosterone levels. | 1a |
Testosterone gels and long-acting injectable TU represent T preparations with the best safety profile. | 1a |
Gonadotropins treatment can be used to restore fertility in men with secondary hypogonadism. | 1a |
Recommendations | Strength rating |
Treat, when indicated, organic causes of hypogonadism (e.g., pituitary masses, hyperprolactinemia, etc). | Strong |
Improve lifestyle and reduce weight (e.g., obesity); withdraw, when possible, concomitant drugs that can impair testosterone production; treat co-morbidity before starting testosterone therapy. | Weak |
Fully inform patients about expected benefits and adverse effects of any treatment option. Select the testosterone preparation in a joint decision process, only with fully informed patients. | Strong |
The aim of testosterone therapy is to restore serum testosterone concentration to the therapeutic range for young men. | Weak |
Use testosterone gels rather than long-acting depot administration when starting initial treatment, so that therapy can be adjusted or stopped in the case of treatment-related adverse effects. | Weak |
3.7. Safety and follow-up in hypogonadism management
3.7.1. Hypogonadism and fertility issues
The aim of pharmacological management of hypogonadism is to increase testosterone levels. The first choice is to administer exogenous testosterone. However, while exogenous testosterone has a beneficial effect on the clinical symptoms of hypogonadism, it inhibits gonadotropin secretion by the pituitary gland, resulting in impaired spermatogenesis and sperm cell maturation [142]. Therefore, testosterone therapy is contraindicated in hypogonadal men seeking fertility treatment [104]. When secondary hypogonadism is present, gonadotropin therapy may maintain normal testosterone levels and restore sperm production [5].
3.7.2. Male breast cancer
In vitro and in vivo studies have clearly documented that breast cancer growth is significantly influenced by testosterone and/or by its conversion to E2 through different mechanisms and pathways [143]. Accordingly, the use of SERMs still represents an important therapeutic option in the management of this cancer [143]. No information is available on the role of testosterone therapy in patients successfully treated for male breast cancer; therefore, treated and active male breast cancer should be recognised as absolute contraindications for testosterone therapy.
3.7.3. Lower urinary tract symptoms/benign prostatic hyperplasia
Based on the assumption that prostate growth is dependent on the presence of androgens, historically testosterone therapy has raised some concerns regarding the possibility of aggravating LUTS in patients affected by benign prostatic hyperplasia (BPH) associated with prostate enlargement [100,144]. However, pre-clinical and clinical data have indicated that low rather than high androgen levels may decrease bladder capacity, alter tissue histology and decrease the ratio of smooth muscle to connective tissue, thus impairing urinary dynamics [100,144].
A trial of 60 patients undergoing testosterone therapy for six months showed no significant differences in post-void residual urine and prostate volume, while storage symptoms as measured by IPSS significantly improved, despite an increase in prostate-specific antigen (PSA) level [145]. A larger pre-treatment prostate volume was a predictive factor of improvement in LUTS. Similarly, a placebo controlled RCT including 120 hypogonadal (total testosterone < 12 nmol/L) men with MetS waitlisted for BPH surgery, showed that testosterone therapy did not result in a difference in in LUTS severity compared to placebo. Conversely, an improvement in ultrasound markers of inflammation in the expression of several pro-inflammatory genes was found in the treatment active arm [146]. A long-term study of 428 men undergoing testosterone therapy for 8 years demonstrated significant improvements in IPSS, no changes max flow rate (Qmax) and residual urine volume, but also a significant increase in prostate volume [147]. Similar data from the Registry of Hypogonadism in Men (RHYME), including 999 patients with a follow-up of 3 years, did not document any significant difference in PSA levels or total IPSS in men undergoing testosterone therapy, compared to untreated patients [148]. Similar results were reported in an Italian registry (SIAMO-NOI), collecting data from 432 hypogonadal men from 15 centres [149]. Meta-analyses have not found significant changes in LUTS between patients treated with testosterone or placebo [150-156]. According to the most recent literature, there are no grounds to discourage testosterone therapy in hypogonadal patients with BPH/LUTS and there is evidence of limited benefit from androgen administration. The only concern is related to patients with severe LUTS (IPSS > 19), as they are usually excluded from RCTs, therefore limiting the long-term safety data of testosterone therapy in this specific setting [100].
3.7.4. Prostate cancer (PCa)
A considerable number of observational studies have failed to demonstrate any association between circulating higher testosterone levels and PCa [157]. In contrast, studies investigating the relationship between low levels of testosterone and risk of PCa have found that men with very low levels of fT have a reduced risk of developing low-to-intermediate-grade PCa, but have a non-significantly increased chance of developing high-grade PCa [157]. This peculiar pattern was also reported in trials such as the Health Professionals Follow-up Study, the Prostate Cancer Prevention Trial (PCPT) and the Reduction by Dutasteride of Prostate Cancer Events (REDUCE), with varying magnitudes of significance [158].
The most recent meta-analysis, including 27 placebo-controlled, RCTs, found no evidence of increased PSA levels following testosterone therapy for one year. When considering 11 studies reporting on the occurrence of PCa, the meta-analysis found no evidence of increased risk of PCa. However, a 1-year follow-up may be considered too short to draw firm conclusions on the risks of developing PCa. Furthermore, the analysis was restricted to studies with > 1-year follow-up, but no significant changes in PSA levels nor increased risk of PCa were found [151]. After 5-years’ median follow-up in three independent registry studies with > 1,000 patients undergoing testosterone therapy, PCa occurrence remained at all times below the reported incidence rate in the general population [159]. Similar results were reported by a more recent large observational study including 10,311 men treated with testosterone therapy and 28,029 controls with a median follow-up of 5.3 years [160]. The same study, also showed that the risk of PCa was decreased for men in the highest tertile of testosterone therapy cumulative dose exposure as compared with controls [160].
With regards to PCa survivors, safety in terms of the risk of recurrence and progression has not yet been established. Limited data are available in the literature, with most case series not providing sufficient data to draw definitive conclusions (e.g., insufficient follow-up, small samples, lack of control arms, heterogeneity in study population and treatment regimen, etc.) [161]. More recently, a meta-analysis derived from 13 studies including 608 patients, of whom 109 had a history of high-risk PCa, with follow-up of 1-189.3 months [162], suggested that testosterone therapy did not increase the risk of biochemical recurrence, but the available evidence is poor, limiting data interpretation [162]. Similar considerations can be derived from another, larger meta-analysis of 21 studies [163]. It is important to recognise that most of the studies analysed included low-risk patients with Gleason score < 8 [162]. Interestingly, Valderrábano et al., recently described the design of the first RCT which assessed the safety/benefit ratio of testosterone therapy in hypogonadal men successfully treated with prostatectomy for non-aggressive prostate cancer [164]. The study is still ongoing and eligible subjects are randomised to testosterone cypionate (100 mg/week) or placebo for 12 weeks, followed by another 12 weeks.
In conclusion, recent literature does not support an increased risk of PCa in hypogonadal men undergoing testosterone therapy. Although it is mandatory to avoid testosterone administration in men with advanced PCa, insufficient long-term prospective data on the safety of androgen administration in PCa survivors [163], without recurrence should prompt caution in choosing to treat symptomatic hypogonadal men in this setting. Specifically, patients should be fully counselled that the long-term effects of testosterone therapy in this setting are still unknown and requires further investigation. If an occult PCa is not detected before initiation of testosterone therapy, treatment may unmask the cancer detected by an early rise in PSA over 6-9 months of therapy. Due to the lack of strong evidence-based data on safety, the possible use of testosterone therapy in symptomatic hypogonadal men previously treated for PCa should be fully discussed with patients and limited to low-risk individuals.
3.7.5. Cardiovascular Disease
Evidence suggests that hypogonadal men have an increased risk of CVD [80,165]. Whether or not LOH is a cause or a consequence of atherosclerosis has not been clearly determined. Late-onset hypogonadism is associated with CV risk factors, including central obesity, insulin resistance and hyperglycaemia, dyslipidaemia (elevated total cholesterol, LDL-cholesterol, triglycerides and low HDL-cholesterol), pro-thrombotic tendency and chronic inflammatory state [165]. Atherosclerosis is a chronic inflammatory disease, that releases pro-inflammatory cytokines into the circulation, which are known to suppress testosterone release from the HPG axis. Evidence from RCTs of testosterone therapy in men with MetS and/or T2DM demonstrates some benefit in CV risk, including reduced central adiposity, insulin resistance, total cholesterol and LDL-cholesterol and suppression of circulating cytokines [14,24-26,31,165]. However, due to the equivocal nature of these studies, testosterone therapy cannot be recommended for indications outside the specific symptoms.
Published data show that LOH is associated with an increase in all-cause and CVD-related mortality [12,166-169]. These studies are supported by a meta-analysis that concluded that hypogonadism is a risk factor for cardiovascular morbidity [155] and mortality [170]. Importantly, men with low testosterone when compared to eugonadal men with angiographically proven coronary disease have twice the risk of earlier death [165]. Longitudinal population studies have reported that men with testosterone in the upper quartile of the normal range have a reduced number of CV events compared to men with testosterone in the lower three quartiles [166]. Androgen deprivation therapy for PCa is linked to an increased risk of CVD and sudden death [171]. Conversely, two long-term epidemiological studies have reported reduced CV events in men with high normal serum testosterone levels [172,173]. Erectile dysfunction is independently associated with CVD and may be the first clinical presentation in men with atherosclerosis.
The knowledge that men with hypogonadism and/or ED may have underlying CVD should prompt individual assessment of their CV risk profile. Individual risk factors (e.g., lifestyle, diet, exercise, smoking, hypertension, diabetes and dyslipidaemia) should be assessed and treated in men with pre-existing CVD and in patients receiving androgen deprivation therapy. Cardiovascular risk reduction can be managed by primary care clinicians, but patients should be appropriately counselled by clinicians active in prescribing testosterone therapy [106]. If appropriate, they could be referred to cardiologists for risk stratification and treatment of co-morbidity.
No RCTs have provided a clear answer on whether testosterone therapy affects CV outcomes. The TTrial (n=790) in older men [174], the TIMES2 (n=220) [25] and, the BLAST studies in men with MetS and T2DM and the pre-frail and frail study in elderly men - all of 1-year duration and the T4DM 2-year study - did not reveal any increase in Major Adverse Cardiovascular Events (MACE) [25,28,29,174,175]. In this context, MACE is defined as the composite of CV death, non-fatal acute myocardial infarction, acute coronary syndromes, stroke and cardiac failure. Randomised controlled trials between 3 and 12 months in men with known heart disease treated with testosterone have not found an increase in MACE, but have reported improvement in cardiac ischaemia, angina and functional exercise capacity [176-178]. A large cohort study (n=20,4857 men) found that neither transdermal gels or intramuscular testosterone was associated with an increased risk of composite cardiovascular outcome in men with or without prevalent cardiovascular disease (mean follow up 4.3 years) [179]. The European Medicines Agency (EMA) has stated that ‘The Co-ordination Group for Mutual recognition and Decentralisation Procedures-Human (CMDh), a regulatory body representing EU Member States, has agreed by consensus that there is no consistent evidence of an increased risk of heart problems with testosterone in men who lack the hormone (a condition known as hypogonadism). However, the product information is to be updated in line with the most current available evidence on safety, and with warnings that the lack of testosterone should be confirmed by signs and symptoms and laboratory tests before treating men with these drugs [180].
As a whole, as for MACE, current available data from interventional studies suggest that there is no increased risk with up to 3 years of testosterone therapy [181-185]. The weight of the currently published evidence has reported that testosterone therapy in men with diagnosed hypogonadism has neutral or beneficial actions on MACE in patients with normalised testosterone levels. The findings could be considered sufficiently reliable for a 3-year course of testosterone therapy, after which no available study can exclude further or long-term CV events [186,187]. To better clarify testosterone-related CV concerns, a specific long-term (approximately 60 months) pharma-supported multi-centre RCT is underway. The latter study is the first placebo controlled RCT specifically designed to investigate CV risk of testosterone therapy (clinicaltrials.gov: NCT03518034).
3.7.5.1. Cardiac Failure
Testosterone treatment is contraindicated in men with severe chronic cardiac failure because fluid retention may lead to exacerbation of the condition. Some studies including one of 12 months’ duration have shown that men with moderate chronic cardiac failure may benefit from low doses of testosterone, which achieve mid-normal range testosterone levels [177,188,189]. If a decision is made to treat hypogonadism in men with chronic cardiac failure, it is essential that the patient is followed up carefully with clinical assessment and both testosterone and haematocrit measurements on a regular basis. An interesting observation is that untreated hypogonadism increased the re-admission and mortality rate in men with heart failure [190]. Similarly to what is reported for other outcomes (see above), a specific RCT assessing possible advantages of testosterone therapy in patients with heart failure, has been designed and it is currently ongoing [191].
3.7.6. Erythrocytosis
An elevated haematocrit is the most common adverse effect of testosterone therapy. Stimulation of erythropoiesis is a normal biological action that enhances delivery of oxygen to testosterone-sensitive tissues (e.g., striated, smooth and cardiac muscle). Any elevation above the normal range for haematocrit usually becomes evident between 3 and 12 months after testosterone therapy initiation. However, polycythaemia can also occur after any subsequent increase in testosterone dose, switching from topical to parenteral administration and, development of co-morbidity, which can be linked to an increase in haematocrit (e.g., respiratory or haematological diseases).
There is no evidence that an increase of haematocrit up to and including 54% causes any adverse effects. If the haematocrit exceeds 54% there is a testosterone independent, but weak associated rise in CV events and mortality [102,192-194]. Any relationship is complex as these studies were based on patients with any cause of secondary polycythaemia, which included smoking and respiratory diseases. There have been no specific studies in men with only testosterone-induced erythrocytosis.
Three large studies have not shown any evidence that testosterone therapy is associated with an increased risk of venous thromboembolism [195,196]. However, one study showed that an increased risk peaked at 6 months after initiation of testosterone therapy, then declined over the subsequent period [197]. No study reported whether there was monitoring of haematocrit, testosterone and/or E2 levels. High endogenous testosterone or E2 levels are not associated with a greater risk of venous thromboembolism [198]. In one study venous thromboembolism was reported in 42 cases and 40 of these had diagnosis of an underlying thrombophilia (including factor V Leiden deficiency, prothrombin mutations and homocysteinuria) [199]. In a RCT of testosterone therapy in men with chronic stable angina there were no adverse effects on coagulation, by assessment of tissue plasminogen activator or plasminogen activator inhibitor-1 enzyme activity or fibrinogen levels [200]. A meta-analysis of RCTs of testosterone therapy reported that venous thromboembolism was frequently related to underlying undiagnosed thrombophilia-hypofibrinolysis disorders [101]. However, another meta-analysis and systematic review of RCTs found that testosterone therapy was not associated with an increased risk of venous thromboembolism [201]. With testosterone therapy an elevated haematocrit is more likely to occur if the baseline level is toward the upper limit of normal prior to initiation. Added risks for raised haematocrit on testosterone therapy include smoking or respiratory conditions at baseline. Higher haematocrit is more common with parenteral rather than topical formulations. Accordingly, a large retrospective two-arm open registry, comparing the effects of long-acting testosterone undecanoate and testosterone gels showed that the former preparation was associated with a higher risk of haematocrit levels > 50%, when compared to testosterone gels [202]. In men with pre-existing CVD extra caution is advised with a definitive diagnosis of hypogonadism before initiating testosterone therapy and monitoring of testosterone as well as haematocrit during treatment.
Elevated haematocrit in the absence of co-morbidity or acute CV or venous thromboembolism can be managed by a reduction in testosterone dose, change in formulation or if the elevated haematocrit is very high by venesection (500 mL), even repeated if necessary, with usually no need to stop the testosterone therapy.
3.7.7. Obstructive Sleep Apnoea
There is no evidence that testosterone therapy can result in onset or worsening of sleep apnoea. Combined therapy with Continuous Positive Airway Pressure (CPAP) and testosterone gel was more effective than CPAP alone in the treatment of obstructive sleep apnoea [203]. In one RCT, testosterone therapy in men with severe sleep apnoea reported a reduction in oxygen saturation index and nocturnal hypoxaemia after 7 weeks of therapy compared to placebo, but this change was not evident after 18 weeks treatment and there was no association with baseline testosterone levels [204].
3.7.8. Follow-up
Testosterone therapy alleviates symptoms and signs of hypogonadism in men in a specific time-dependent manner. The TTrials clearly showed that testosterone therapy improved sexual symptoms as early as
3 months after initiation [115]. Similar results have been derived from meta-analyses [55,101]. Hence, the first evaluation should be planned after 3 months of treatment. Further evaluation may be scheduled at 6 months or 12 months, according to patient characteristics, as well as results of biochemical testing (see below). Patients at high risk of developing elevated haematocrit should be evaluated every three months during the first year of testosterone therapy and at least every six months thereafter. Accordingly, current guidelines suggest that haematocrit should be maintained below 45% in patients with polycythaemia vera to avoid thromboembolism risk [205]. Similarly, data derived using a multi-institutional database including a large cohort of hypogonadal (total testosterone < 12 nmol/L) men who received testosterone therapy and subsequently did (n=5,887) or did not (n=4,2784) develop polycythaemia (haematocrit > 52%) showed that men who had an increased haematocrit had a higher risk of MACE or venous thromboembolism mostly during the first year of therapy [206]. The risk was even higher when a haematocrit threshold of 54% was considered whilst no risk was observed when a 50% threshold was applied [206]. Table 6 summarises the clinical and biochemical parameters that should be monitored during testosterone therapy.
Trials were designed to maintain the serum testosterone concentration within the normal range for young men (280–873 ng/dL or 9.6-30 nmol/L) [115]. This approach resulted in a good benefit/risk ratio. A similar approach could be considered during follow-up. The correct timing for evaluation of testosterone levels varies according to the type of preparation used (Table 5). Testosterone is involved in the regulation of erythropoiesis [140] and prostate growth [100], hence evaluation of PSA and haematocrit should be mandatory before and during testosterone therapy. However, it is important to recognise that the risk of PCa in men aged < 40 years is low. Similarly, the mortality risk for PCa in men aged > 70 years has not been considered high enough to warrant monitoring in the general population [207]. Hence, any screening for PCa through determination of PSA and DRE in men aged < 40 or > 70 years during testosterone therapy should be discussed with the patients.
Baseline and, at least, annually glyco-metabolic profile evaluation may be a reasonable consideration, particularly in the management of functional hypogonadism. Testosterone therapy may be beneficial for hypogonadal men with low or moderate fracture risk [124]; therefore, dual energy X-ray absorptiometry (DEXA) bone scan may also be considered at baseline and 18-24 months following testosterone therapy, particularly in patients with more severe hypogonadism [124].
Digital rectal examination may detect prostate abnormalities that can be present even in men with normal PSA values. Hence, DRE is mandatory in all men at baseline and is recommended to be performed only annually during testosterone therapy, as long as there is no significant increase in PSA velocity.
The decision to stop testosterone therapy or to perform prostate biopsy due to PSA increase or prostate abnormalities should be based on local PCa guidelines. There is a large consensus that any increase of haematocrit > 54% during testosterone therapy requires therapy withdrawal and phlebotomy to avoid potential adverse effects including venous-thromboembolism and CVD, especially in high-risk individuals. In patients with lower risk of relevant clinical sequelae, the situation can be alternatively managed by reducing testosterone dose and switching formulation along with venesection. A positive family history of venous-thromboembolism should be carefully investigated and the patient counselled with regard to testosterone therapy to avoid/prevent thrombophilia-hypofibrinolysis [101]. Finally, caution should be exercised in men with pre-existing CVD or at higher risk of CVD.
Table 6: Clinical and biochemical parameters to be checked during testosterone therapy
Parameters | Year 1 of treatment | After year 1 of treatment | ||||
Baseline | 3 months | 6 months | 12 months | Annually | 18-24 months | |
Clinical | ||||||
Symptoms | X | X | X | X | X | |
Body Mass Index | X | X | X | |||
Waist circumference | X | X | X | X | ||
Digital rectal examination | X | X | X | |||
Blood pressure | X | X | X | X | ||
Biochemistry | ||||||
PSA (ng/mL) | X | X | X2 | X | X | |
Haematocrit (%) | X | X | X1,2 | X | X | |
Testosterone | X | X | X | X | ||
Lipid and glycaemic profile | X | X | X | |||
Instrumental | ||||||
DEXA | X | X | ||||
1 Population with polycythaemia vera or at high risk of secondary polycythaemia (e.g., sleep apnea, morbidobesity, heavy smokers, chronic obstructive pulmonary disease); 2 Prostate cancer survivors.
3.7.9. Summary of evidence and recommendations on risk factors in testosterone treatment
Summary of evidence | LE |
Testosterone therapy is contraindicated in men with secondary hypogonadism who desire fertility. | 1a |
Testosterone therapy is contraindicated in men with active prostate cancer or breast cancer, as these patients are usually excluded from RCTs. | 1a |
Testosterone therapy does not increase the risk of prostate cancer, but long-term prospective follow-up data are required to validate this statement. | 1a |
The effect of testosterone therapy in men with severe lower-urinary tract symptoms is limited, as these patients are usually excluded from RCTs. | 1a |
There is no substantive evidence that testosterone therapy, when replaced to normal levels, results in the development of major adverse cardiovascular events. | 1a |
There is no evidence of a relationship between testosterone therapy and mild, moderate or CPAP-treated severe sleep apnoea. | 1b |
Recommendations | Strength rating |
Fully counsel symptomatic hypogonadal men who have been surgically treated for localised prostate cancer (PCa) and who are currently without evidence of active disease considering testosterone therapy, emphasising the benefits and lack of sufficient safety data on long-term follow-up. | Weak |
Restrict treatment to patients with a low risk for recurrent PCa (i.e., pre-operative PSA | Weak |
Advise patients that safety data on the use of testosterone therapy in men treated for breast cancer are unknown. | Strong |
Assess cardiovascular risk factors before commencing testosterone therapy. | Strong |
Assess men with known cardiovascular disease (CVD) for cardiovascular symptoms before testosterone therapy and with close clinical assessment and evaluation during treatment. | Strong |
Treat men with hypogonadism and pre-existing CVD, venous-thromboembolism or chronic cardiac failure, who require testosterone therapy with caution, by careful clinical monitoring and regular measurement of haematocrit (not exceeding 54%) and testosterone levels. | Weak |
Exclude a family history of venous-thromboembolism before starting testosterone therapy. | Strong |
Monitor testosterone, haematocrit at three, six and twelve months after testosterone therapy initiation, and thereafter annually. A haematocrit > 54% should require testosterone therapy withdrawal and phlebotomy. Re-introduce testosterone therapy at a lower dose once the haematocrit has normalised and consider switching to topical testosterone preparations. | Strong |
Evaluate patients with polycythaemia vera and those with a higher risk of developing elevated haematocrit every three months during the first year of testosterone therapy, and at least every six months thereafter. | Strong |
Evaluate total PSA in PCa survivors at three, six and twelve months during the first year of testosterone therapy, and annually thereafter. | Strong |
*As for EAU risk groups for biochemical recurrence of localised or locally advanced prostate cancer (see EAUProstate Cancer Guidelines, 2023)